Abstract
Objective
Clinical presentation and physical signs may be unreliable in the diagnosis of stercoral colitis (SC). This study evaluates the value of computed tomography (CT) in distinguishing fatal from non-fatal SC.
Materials and Methods
Ten patients diagnosed as SC were obtained from inter-specialist conferences. Additional 13 patients with suspected SC were identified via the Radiology Information System (RIS). These patients were divided into two groups; fatal and non-fatal SCs. Their CT images are reviewed by two board-certified radiologists blinded to the clinical data and radiographic reports.
Results
SC occurred in older patients and displayed no gender predisposition. There was significant correlation between fatal SC and CT findings of dense mucosa (p = 0.017), perfusion defects (p = 0.026), ascites (p = 0.023), or abnormal gas (p = 0.033). The sensitivity, specificity, and accuracy of dense mucosa were 71%, 86%, and 81%, respectively. These figures were 75%, 79%, and 77% for perfusion defects; 75%, 80%, and 78% for ascites; and 50%, 93%, and 78% for abnormal gas, respectively. Each CT sign of mucosal sloughing and pericolonic abscess displayed high specificity of 100% and 93% for diagnosing fatal SC, respectively. However, this did not reach statistical significance in diagnosing fatal SC.
Fecaloma impaction associated stercoral colitis (SC) can result in catastrophic complications such as perforation and fecal peritonitis that may become life threatening (1-4), with a mortality rate of 32-57% (5). The most vital aspect of SC diagnosis is based on patient history (old age, chronic constipation, inactive status, use of opioids or Non-steroid anti-inflammatory drugs, and medical comorbidity), clinical presentation of peritonitis, and imaging findings of impacted fecalomas with abnormal radiographic findings (6). Neither physical examination nor laboratory tests alone are reliable to indicate the presence of the disease (7-9).
A positive diagnosis of SC can be confirmed surgically and histopathologically (10). Important surgical findings include fecalomas and the associated mucosal ulcers. Occasionally, multiple ulcers are discovered (10), these ulcers are well-demarcated with sharp margins and without undermining. Nonspecific inflammatory changes of mononuclear cells in the lamina propria can also be expected (11). Crypt abscess, transmural necrosis, and stercoral perforation may or may not occur.
Several helpful CT findings relating to SC have been reported (7, 11). However, no study has yet compared the CT findings of fatal SC to those of non-fatal SC. This study retrospectively attempts to refine the diagnostic value of CT and discriminate CT findings of fatal SC from those of non-fatal SC.
This is a retrospective study and approval has been obtained from the institutional review board of the hospital in question. Between November 2002 and August 2009, ten patients with surgically and pathologically confirmed SC were recruited from inter-specialist conferences (7). From Jan 2004 to April 2010, another 13 patients with suspected SC as shown by CT reports were identified via RIS. These 13 patients exhibit a positive history (old age, chronic constipation, inactive status, use of opioids or NSAIDs, and medical comorbidity). On admission, the patients presented with constipation and abdominal pain. Each CT showed a fecalomas-impacted colon with features of SC. None of these patients displayed occlusion of either the mesenteric artery or vein. For this study, a fatal SC is defined as one that may cause sepsis-related death to a patient who otherwise has no other discernible infection foci during the course of hospitalization.
The patients used in this study are 13 men and ten women with ages ranging from 34 to 93 years (mean age, 73 years). Eleven patients (ten from an inter-specialist conference and one from RIS) underwent colectomies and histopathological studies; the other 12 were diagnosed based on CT findings and discharge notes. Patients are divided into two groups: fatal (n = 8) and non-fatal (n = 15) SC. No statistical difference in age or gender is identified between the groups. Of the eight patients with fatal SC, seven (87.5%) underwent surgical colectomies. Of the 11 patients who underwent surgical intervention, four were non-fatal (36.4%). The mean time between CT and surgical colectomy is seen to be 31 hours (two hours to seven days).
Due to the retrospective nature of this study, a uniform protocol could not be achieved in the relevant tertiary-care hospital with multiple CT machines. However, CT examinations were mostly performed by a four-detector CT scanner (LightSpeed QX/i Scanner, General Electric Medical Systems, Milwaukee, WI, USA). Helical CT images were acquired using 5-mm slice collimation, a reconstruction interval of 5 mm, pitch of 1.5-2, 120 kV, and 200-240 mA. Intravenous (IV) contrast agent (100 mL) was routinely used in accordance with departmental protocols.
Two board-certified radiologists with expertise in abdominal imaging reviewed the CT images in the context of the hospital's picture archiving and communication system. These reviewers were blinded to the official CT reports as well as to the surgical and pathologic findings. They were requested to determine the presence or absence of the CT features of SC. For further analysis, disagreements were resolved through discussion involving a third radiologist's opinion until consensus was achieved.
Individual CT signs are defined as follows: colon dilatation, the colon segment proximal to obstruction is distended with a cross-sectional luminal diameter > 6 cm; wall thickening, colon wall thickness is > 3 mm at stool impaction; dense mucosa, increased mucosal lining density on the pre-contrast CT; mucosal sloughing, mucosa dislodged into the lumen; perfusion defect, discontinuity of the colon mucosal enhancement or apparently decreased enhancement compared with adjacent bowel loops; pericolonic stranding, increased streaks of pericolonic fat; pericolonic abscess, fluid collection or mottled substances around the damaged colon; ascites, extra-luminal fluid in the peritoneal space; abnormal gas, gas migrating into or beyond the colon wall.
Dense mucosal lining conforming to the colon wall is differentiated from calcified fecaloma, which presents as clustered masses in the lumen with a dense rim and gas in between (12).
The chi-squared test and Fisher's exact test are used to determine the relation of the categorical data (gender and individual CT sign) to the final diagnosis. The Mann-Whitney U-test is used to examine the relationship between the continuous variables of patients' age and final diagnosis. Kappa statistics are used to assess the inter-observer agreement. Statistical significance is deemed to occur when a p value is less than 0.05.
The percentage of each positive CT finding for SC, listed in decreasing order, is as follows: wall thickening (56.5%, n = 13), pericolonic stranding (52.2%, n = 12), perfusion defect (40.9%, n = 9), ascites (39.1%, n = 9), dense mucosa (33.3%, n = 7), colon dilatation (21.7%, n = 5), abnormal gas (21.7%, n = 5), pericolonic abscess (8.7%, n = 2), and mucosal sloughing (4.3%, n = 1).
The inter-observer agreement is shown in Table 1. Agreement is seen to be good-to-excellent for all signs except wall thickening and perfusion defects (Fig. 1). Disagreement occurs with respect to wall thickening in nine patients, perfusion defects in five, dense mucosa (Fig. 2) in three, pericolonic stranding (Fig. 2) in three, mucosal sloughing (Fig. 3) in one, and abnormal gas (Fig. 4) in one. Consensus among the examiners was generally achieved following an open discussion and the opinion of a third radiologist when necessary.
Data for the CT findings are presented in Tables 1 and 2. Fatal SC exhibits significant correlation with dense mucosa (p = 0.017), perfusion defect (p = 0.026), ascites (p = 0.023), and abnormal gas (p = 0.033). The Odds Ratio for dense mucosa, perfusion defect, ascites, and abnormal gas is observed to be 15, 11, 12, and 14, respectively. Of these four variables, the individual accuracy is approximately 80 percent, and a relative higher specificity of 93.3 percent is identified in abnormal gas.
Each CT sign of mucosal sloughing and abscess (Fig. 4) displays high specificity (100.0% and 93.3%, respectively). However, the sensitivity and accuracy is seen to be low for fatal SC and does not reach statistical significance.
The main question addressed by this study is whether CT is valuable in predicting fatal SC. The study shows that the CT signs of dense mucosa, perfusion defect, ascites, and abnormal gas are statistically different between fatal and non-fatal SC, indicating the value of CT.
Dense mucosa resulting from mucosal hemorrhage has been reported to be a sign of ischemic bowels (13, 14). The CT appearance and density of the intramural hemorrhage of the intestine depends on the age of the hemorrhage (15). A perfusion defect can indicate a change in status, from ischemia to infarct (4, 16), as well as the occurrence of perforation (17-19). It is possible that this is the result of a subtle and localized change, but the inter-observer agreement for perfusion defects is fair. Among these four statistically significant CT signs, the inter-observer agreement is highest for ascites. This indicates that ascites is a more objective sign that is harder to dispute. Ascites could be induced by hypoalbunemia related to severe systemic inflammation, or cardio-respiratory co-morbidities. This implies that ascites is an ominous sign of fatal SC. Abnormal gas related to SC is seen to be occasionally inconspicuous and CT is identified as the most reliable modality to depict the extra-luminal gas (4). In this study, it presents as a small gas bubble that appears on CT scans in the proximity of the colon wall, entrapped in the colon wall (pneumo-intestinalis coli), or confined inside the mesocolon (pneumo-mesocolon). These bubbles are often radiographically imperceptible and differ from gastroduodenal perforation. The location of pneumoperitoneum solely in the pelvis is frequently due to colonic perforation (18, 20-23). A colon perforator may be sealed off by fecaloma or inflammatory fibrin, as noted surgically.
Each CT sign of mucosal sloughing and pericolonic abscess displays high specificity, but low sensitivity. This indicates that SC may progress and become fatal in a subject showing CT signs of mucosal sloughing or pericolonic abscess. The mucosal sloughing shown in this study is delineated as a narrow base of a mucosal flap in the distended colon that differs from the colon haustra. In fact, the definition of pericolonic abscess formation used in this study is met by perforation of the colon with fecal material passing through the colon wall and confined in the pericolonic area.
Colon dilatation is not frequently observed, and does not correlate well with fatal SC. This may be due to a lack of colon dilatation, causing clinicians overlook the severity of SC. Wall thickening of the colon is most frequently seen and indicates that colitis already exists (24). This may be focal and subtle, resulting in slight inter-observer agreement. Pericolonic stranding is the second most frequently observed CT sign of SC. Pericolonic stranding implies the transmural extension of the disease and is likely associated with the pericolonic inflammation and edema that causes the intolerable abdominal pain experienced by these patients.
A review of the literature documentation (25-27) shows that SC occurs mostly at the sigmoid colon in the study. Possible reasons for this include the sigmoid colon being the narrowest segment of the entire colon, a prolonged stay of fecaloma with resultant solid consistency, and the precarious blood supply region known as Sudeck's point (28). SC is a spectrum of colonic disease, ranging from simple fecaloma impaction and colon ulcers to fatal colonic perforation (8). The treatment principle is early extraction of the impacted fecaloma to relieve colon pressure and lessen the likelihood of ulceration (11), along with appropriate treatment of any co-morbid medical condition (26). Active treatment of constipation and removal of fecaloma is a preventative policy (2, 8).
Of the 23 patients in this study, 12 (52.2%) were relieved by a non-surgical method, such as a cathartic or digital disimpaction, and were discharged uneventfully. Eleven (47.8%) deteriorated clinically and required surgical intervention. Fatal SC exhibits a statistical correlation with surgery. Severity of the SC is the presumed reason that seven patients eventually died despite surgery. This relationship between mortality and surgery does not imply causality; rather, the sicker patients were more likely to be treated surgically.
The present study has several limitations: small sample size, co-mortality, not all patients having had a histopathological study, and the study's retrospective nature. The small sample size is related to the low incidence and underestimation of the disease (10). The incidence of perforated stercoral ulcer at autopsy ranges from 0.04% to 2.3%. Stercoral perforation of the colon is found in 0.5% of all surgical colorectal procedures, 1.2% of all emergency colorectal procedures, and 3.2% of all colonic perforations (10). Patients presenting SC are normally those with medical comorbidity and advanced age (6). Emergent surgery and occasional fecal peritoneal contamination due to perforation are also risk factors for mortality (8). Since most patients experience clinical improvement after disimpaction, not all patients in this study underwent surgical colon resection. It is acknowledged that endoscopic findings of stercoral ulcers would be satisfactory evidence of SC in constipated patients. A refined prospective evaluation of the diagnostic value of CT for SC should be carried out on a larger subject population with endoscopic finding also added as another inclusion criterion. Due to the retrospective nature of this study, some important clinical data and images were unavailable.
Figures and Tables
Fig. 1
Enhanced abdomen CT for 71-year-old woman with non-fatal SC demonstrating wall thickening (arrowhead) at recto-sigmoid colon, discontinuation of enhanced mucosa that indicates perfusion defect (white arrow). Regional ascites accumulation (black arrow) is also seen. SC = stercoral colitis
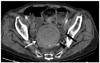
Fig. 2
Fatal stercoral colitis in 79-year old woman.
A. Un-enhanced CT showing dense rim conforming to wall of sigmoid colon impressed as dense mucosa (white arrow). Increased streaky pericolic infiltration (arrowhead) is also seen. B. Note presence of mucosal hemorrhage and marked submucosal congestion of tissue specimen.
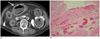
Fig. 3
Enhanced abdomen CT for 88-year-old woman with fatal SC revealing mucosal slip (arrow) sloughing out from wall of distended colon. Surgical resection confirmed SC with gangrene mucosa in sloughing. SC = stercoral colitis
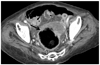
Fig. 4
Enhanced CT for 87-year-old man with non-fatal SC revealing confined mottled substance abutting sigmoid colon (arrow), indicative of perisigmoid abscess formation. Extra-luminal gas (white arrowhead) around the sigmoid colon is also seen. SC = stercoral colitis
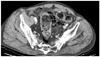
Table 1
Correlation of Individual CT Signs with Fatal SC

Note.- *Dense mucosa is evaluated with pre-contrast CT scanning in 21 of patients who had undergone scanning of lower abdomen, †Perfusion defect is evaluated with contrast-enhanced CT scanning in 22 of patients, ‡By Fisher's exact test. NA = not applicable, n = exam number, SC = stercoral colitis, OP = operation
Acknowledgments
We want to thank all the staff at the Chang Guang Memorial Hospital involved in the management of these cases. We also thank the Bio-statistic Center affiliated with Chang Guang Memorial University for providing technical support.
References
1. Yano T, Wakabayashi H, Yachida S, Okano K, Izuishi K, Suzuki Y. A stercoral perforation of the colon with an obvious faecal mass diagnosed by computed tomography. ANZ J Surg. 2008. 78:214–215.
2. Wald A. Management and prevention of fecal impaction. Curr Gastroenterol Rep. 2008. 10:499–501.
3. Sharma M, Agrawal A. Case report: Stercoral sigmoid colonic perforation with fecal peritonitis. Indian J Radiol Imaging. 2010. 20:126–128.
4. Zissin R, Hertz M, Osadchy A, Even-Sapir E, Gayer G. Abdominal CT findings in nontraumatic colorectal perforation. Eur J Radiol. 2008. 65:125–132.
5. Serpell JW, Nicholls RJ. Stercoral perforation of the colon. Br J Surg. 1990. 77:1325–1329.
6. Haddad R, Bursle G, Piper B. Stercoral perforation of the sigmoid colon. ANZ J Surg. 2005. 75:244–246.
7. Wu CH, Wang LJ, Wong YC, Huang CC, Chen CC, Wang CJ, et al. Necrotic stercoral colitis: importance of computed tomography findings. World J Gastroenterol. 2011. 17:379–384.
8. Ouaïssi M, Sielezneff I, Benoist S, Pirró N, Cretel E, Chaix JB, et al. Lethal fecaloma. J Am Geriatr Soc. 2007. 55:965–967.
9. Rozenblit AM, Cohen-Schwartz D, Wolf EL, Foxx MJ, Brenner S. Case reports. Stercoral perforation of the sigmoid colon: computed tomography findings. Clin Radiol. 2000. 55:727–729.
10. Maurer CA, Renzulli P, Mazzucchelli L, Egger B, Seiler CA, Büchler MW. Use of accurate diagnostic criteria may increase incidence of stercoral perforation of the colon. Dis Colon Rectum. 2000. 43:991–998.
11. Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005. 184:1189–1193.
12. Kim MJ, Park SH, Lee SS, Byeon JS, Choi EK, Kim JH, et al. Efficacy of barium-based fecal tagging for CT colonography: a comparison between the use of high and low density barium suspensions in a Korean population - a preliminary study. Korean J Radiol. 2009. 10:25–33.
13. Frager DH, Baer JW. Role of CT in evaluating patients with small-bowel obstruction. Semin Ultrasound CT MR. 1995. 16:127–140.
14. Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, et al. CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009. 192:408–416.
15. Boudiaf M, Soyer P, Terem C, Pelage JP, Maissiat E, Rymer R. Ct evaluation of small bowel obstruction. Radiographics. 2001. 21:613–624.
16. Rha SE, Ha HK, Lee SH, Kim JH, Kim JK, Kim JH, et al. CT and MR imaging findings of bowel ischemia from various primary causes. Radiographics. 2000. 20:29–42.
17. Lohrmann C, Ghanem N, Pache G, Makowiec F, Kotter E, Langer M. CT in acute perforated sigmoid diverticulitis. Eur J Radiol. 2005. 56:78–83.
18. Miki T, Ogata S, Uto M, Nakazono T, Urata M, Ishibe R, et al. Multidetector-row CT findings of colonic perforation: direct visualization of ruptured colonic wall. Abdom Imaging. 2004. 29:658–662.
19. Kim SW, Shin HC, Kim IY, Kim YT, Kim CJ. CT findings of colonic complications associated with colon cancer. Korean J Radiol. 2010. 11:211–221.
20. Rubesin SE, Levine MS. Radiologic diagnosis of gastrointestinal perforation. Radiol Clin North Am. 2003. 41:1095–1115. v
21. Yeung KW, Chang MS, Hsiao CP, Huang JF. CT evaluation of gastrointestinal tract perforation. Clin Imaging. 2004. 28:329–333.
22. Hainaux B, Agneessens E, Bertinotti R, De Maertelaer V, Rubesova E, Capelluto E, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. AJR Am J Roentgenol. 2006. 187:1179–1183.
23. Maniatis V, Chryssikopoulos H, Roussakis A, Kalamara C, Kavadias S, Papadopoulos A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging. 2000. 25:373–379.
24. Yoo HJ, Kim SH, Lee JM, Kim MA, Han JK, Choi BI. The association of anisakiasis in the ascending colon with sigmoid colon cancer: CT colonography findings. Korean J Radiol. 2008. 9:Suppl. S56–S60.
25. Sood BP, Rezai S, Parker D. Unusual radiological appearance of a faecaloma. Australas Radiol. 2007. 51 Spec No:B161–B164.
26. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. Management of patients with stercoral perforation of the sigmoid colon: report of five cases. World J Gastroenterol. 2006. 12:500–503.
27. Patel VG, Kalakuntla V, Fortson JK, Weaver WL, Joel MD, Hammami A. Stercoral perforation of the sigmoid colon: report of a rare case and its possible association with nonsteroidal anti-inflammatory drugs. Am Surg. 2002. 68:62–64.
28. Chen JH, Shen WC. Rectal carcinoma with stercoral ulcer perforation. Hepatogastroenterology. 2000. 47:1018–1019.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download