Abstract
Objective
To determine the prevalence of thyroid incidentalomas detected by time-resolved magnetic resonance angiography (TRMRA) and to evaluate their clinical significance by using an ultrasonographic (US) and cytologic correlation.
Materials and Methods
We retrospectively reviewed 2010 consecutive TRMRA examinations performed at our institution between August 2006 and April 2010. The TRMRA findings of thyroid incidentalomas were analyzed according to location, size, as well as vascularity, and were correlated with the US findings and cytologic results. Each nodule was classified as suspiciously malignant, indeterminate or probably benign according to the US criteria recommended by the Korean Society of Thyroid Radiology.
Results
A total of 102 incidentalomas were detected in 90 of 2010 patients (5%). TRMRA showed homogenous hypervascularity in 48 (47%), inhomogeneous hypervascularity in 46 (45%), and hypovascularity in 8 (8%) thyroid nodules. At follow-up study, out of 26 patients with 30 incidentalomas on TRMRA, 27 nodules were identified on US. Of the 27 nodules, 24 (89%) nodule were classified as indeterminate, two (7%) as probably benign, and one (4%) as suspiciously malignant. Among the 16 nodules with available cytopathologic results, 14 (82%) were benign, one (6%) was indeterminate, and one (6%) was malignant.
A thyroid incidentaloma is defined as a thyroid nodule initially discovered by imaging modalities performed for an unrelated purpose. The incidence of thyroid incidentalomas continues to rise along with the liberal use and technical development of various imaging modalities, such as US, computed tomography, and magnetic resonance (MR) imaging (1). Contrast enhanced MR angiography has been incorporated into the routine examination for neck vessels work up due to its high spatial resolution, signal-to-noise ratio, and contrast-to-noise ratio (2, 3). In particular, with the advent of high performance 3T imaging, time-resolved MR angiography (TRMRA) has recently become popular as it provides both high temporal resolution and spatial resolution (4). Using TRMRA, one can obtain images at multiple time points, which means that theoretically, we could detect any hypervascluar neck tumors within the field of view, which can be detected at various time points during carotid circulation. Accordingly, in daily clinical practice, thyroid incidentalomas have increasingly been detected by TRMRA. However, to the best of our knowledge, there has only been a single, small-scale report considering the frequency of occurrence and the radiologic-pathologic correlation for thyroid incidentalomas identified by TRMRA (5).
The objectives of our study were to determine the prevalence of thyroid incidentalomas found during TRMRA examinations and to evaluate the clinical significance of thyroid incidentalomas detected by TRMRA according to the relationship with US findings and cytology results.
A total of 2010 subjects underwent TRMRA at 3T between August 2006 and April 2010. The subjects consisted of 1042 men and 968 women aged from 30-88 years (mean age, 64.3 ± 10.8 years). All examinations were performed as part of a preventive health screening or for a risk evaluation procedure for cerebrovascular stroke. Twenty-six patients (11 men and 15 women; mean age 63.7 ± 8.8 years; range 49-82 years) underwent further US examination of the thyroid. Median interval time between TRMRA and thyroid US was 7 days. Of the 26 patients, 15 underwent an aspiration biopsy under US guidance and one patient underwent surgery without aspiration biopsy. Five patients did not require an aspiration biopsy based on the assessment of their thyroid US. Of the 5 patients who did not undergo any further workup, one patient refused the aspiration biopsy and 4 were lost to follow-up.
Our study was approved by our institutional review board. As the patients' data were evaluated retrospectively and anonymously, no written informed consent was necessary.
All MR examinations were obtained using a 3T MR scanner (Signa HDx; GE Healthcare, Milwaukee, WI, USA) with an 8-channel head coil. A typical injection protocol consisted of a bolus injection of 10 mL of gadobutrol (Gadovist® 1.0, Bayer Schering Pharma AG, Berlin, Germany) at a rate of 2.0 mL/s, followed by flushing with 35 mL of saline bolus at the same rate. TRMRA was acquired with a three-dimensional gradient echo sequence using a time-resolved method (Time-Resolved Imaging of Contrast Kinetics, TRICKS). The acquisition parameters for TRMRA were as follows: TR/TE of 2.704/0.74 ms, flip angle of 20°, matrix 320 × 192, slice thickness of 1.6 mm, field of view of 320 mm, and a bandwidth of 83.33 kHz. The three-dimensional volume acquisitions were coronally oriented with section coverage from the aortic arch to the circle of Willis, and laterally to both subclavian arteries. Moreover, the volume acquisitions were conducted in 13 temporal phases with a temporal resolution time of 2.4 seconds and a total scan time of approximately 41 seconds. Each three-dimensional image set was viewed as coronal maximum intensity projections (MIP).
Two radiologists (W.J.M and N.C, with 11 and 8 years of experience of thyroid ultrasound and fine needle aspiration [FNA], respectively) performed all ultrasonographic (US) examinations and FNA procedures. All US examinations were performed using a HDI 5000 (ATL, Ultrasound, Bothell, WA) or an IU 22 (Philips Medical Systems, Bothell, WA, USA) instrument equipped with either a 7-15 MHz or a 5-12 MHz linear array transducer.
Aspiration biopsies were performed by the same radiologist who performed the US examination using a free-hand technique with a 22 gauge needle and a 5-mL disposable plastic syringe. Each nodule was aspirated at least twice.
Time-resolved MR angiography and US images of thyroid incidentalomas were retrospectively reviewed by two radiologists (W.J.M. and N.C), who were blinded to the cytopathology results. If there was disagreement, the radiologists reached a consensus after a discussion.
On TRMRA, thyroid incidentalomas were analyzed according to their maximal diameter and vascularity. The vascularity of a thyroid nodule was categorized as homogenously hypervascular (an area of high signal intensity more than 90%), inhomogenously hypervascular (an area of high signal intensity more than 10% but less than 90%), or hypovascular (an area of low signal intensity more than 90%), and compared with the vascularity of thyroid parenchyma. In patients with cytolopathological results, time-signal intensity curves from the TRMRA were generated for each thyroid nodule by plotting signal intensity of the region of interest (ROI) at the 13 temporal phases.
For the incidentalomas in patients who underwent thyroid US, the US images were reviewed and the incidentalomas were matched to the corresponding nodules on US. US characteristics and US diagnoses were prospectively recorded and classified according to the standardized lexicon. Each nodule was classified as suspiciously malignant, indeterminate or probably benign, as recommended by the Korean Society of Thyroid Radiology (6, 7). Findings suspicious for malignancy on US were defined as having at least one of the following features: taller than wide shape, spiculated margin, marked hypoechogenicity, or the presence of micro- or macrocalcifications. Probably benign findings were defined by the presence of a spongiform nodule, or of a predominantly cystic mass with a comet-tail artifact. Indeterminate findings were defined as all US findings other than those indicating probable benignity or malignancy of a nodule.
Aspiration cytologies were categorized as malignant, benign, indeterminate or nondiagnostic. The "indeterminate" category encompassed those samples demonstrating hypercellularity suggestive of a follicular neoplasm, and/or atypical cytoarchitectural features suggestive of, but not diagnostic for malignancy.
A total of 102 thyroid incidentalomas were detected in 90 (40 males and 50 females; mean age 65.2 ± 10.1 years, 41-88 years) of the 2010 patients evaluated, a prevalence of 4.5%. The mean size of the 102 incidentalomas was 11.3 ± 6.5 mm (range 3.4-35.0 mm). Forty-eight nodules (47.1%) showed homogenous hypervascularity (Fig. 1A); 46 nodules (45.1%) showed inhomogenous hypervascularity (Fig. 2A); and eight nodules (7.8%) showed hypovascularity (Fig. 3A).
Of the 30 incidentalomas (80%) found in the 26 patients who underwent subsequent thyroid US, 27 were identified by US. The mean size was 12.8 ± 7.8 mm (range 5.0-35.0 mm). Most incidetalomas (24/27, 88.9%) were classified as indeterminate on US (Figs. 1, 2), but two nodules (7.4%) were classified as probably benign (Fig. 3), and one nodule (3.7%) was classified as suspiciously malignant (Fig. 4). Of the 27 incidetalomas identified on US, 26 nodules were hypervascular (96.3%). Of the 26 hypervascular nodules, 24 (92.3 %) were classified as indeterminate on thyroid US.
Of the 27 nodules identified on US, 17 were subsequently aspirated under US guidance. The cytology results were benign in 13 nodules, indeterminate (follicular neoplasm of Hurthle cell variant) in one nodule, malignant (papillary carcinoma) in one nodule, and of inadequate cytology in two nodules. The two nodules with inadequate cytology were ultimately diagnosed as being benign following a thyroidectomy. The correlation between vascularity on TRMRA, US and cytopathology diagnoses are provided in Table 1. Two incidental nodules with inadequate cytology were initially categorized as indeterminate on the basis of US criteria.
A comparative analysis of the dynamic curves of the nonmalignant and malignant nodules is displayed in Fig. 5. The two dynamic curves did not exhibit any visible differences.
The widened use of cross-sectional imaging studies such as CT or MRI, and their high resolution and sensitivity, have led to an increase in the incidental detection of thyroid nodules (1). However, few CT- or MRI-based studies have reported on the prevalence of incidental thyroid nodules. Youserm et al (8) reported that thyroid incidentalomas were present in 15.6% of 123 CT scans and 108 MRI examinations of the head and neck. A more recent study (9) reported thyroid incidentalomas in 16.8% of 734 neck CT scans. Meanwhile, a study of 624 TRMRA examinations (5) reported a thyroid incidentaloma prevalence of 7.8%, compared to 4.5% in our study. The lower prevalence may have been due to the intrinsically lower resolution of MR angiography compared with CT and MRI, and partly due to differences in our inclusion criteria. Our study included a substantial number of healthy subjects, for whom TRMRA was performed as part of a health screening procedure. Conversely, our study included the largest population (2010 cases) ever evaluated and thus the prevalence of 4.5% from our study may be more accurate than other studies.
While most thyroid incidentalomas are benign, a small proportion (5-10%) are malignant (10). A previous study suggested a significant prevalence of malignancy of up to 8.3% among hyperenhancing thyroid incidentalomas on TRMRA (5). In contrast, in our study, only one of the 17 (5.9%) nodules with cytopathologic results was malignant, which was a lower incidence than was found in the previous study (5). The true incidence rate of malignancy among incidentalomas detected on TRMRA could not be determined accurately because previous studies have only had a small fraction of incidentalomas that were histologically examined: 1.9% (12/624) in the study by Lohan et al., and 0.8% (17/2010) in our study. The results from these two studies suggest that the cancer prevalence for thyroid incidentalomas found by TRMRA may be similar to that for thyroid incidentalomas found by other imaging modalities (11).
Higher intrinsic vascularity compared with the adjacent thyroid parenchyma is the most common vascular pattern exhibited by papillary thyroid carcinoma (12). Several previous studies using color Doppler US have reported that a prominent internal or central blood flow within a thyroid nodule is indicative of malignancy (13-17). However, more recent studies have argued that more than 50% of hypervascular nodules are benign (15, 18). Our results corroborate with these more recent observations in that most thyroid incidentalomas (92.2%) were hypervascular but were considered as being benign by either US or fine needle aspiration biopsy (FNAB). Therefore, we suggest that nodule hypervascularity is a nonspecific finding and does not aid pathologic discrimination.
As expected from the angiogenic nature of all malignant tumors, hypovascular papillary carcinomas are uncommon (12). In our study, only one of the two hypovascular nodules identified by TRMRA was further examined with US, the results of which suggested the nodule was benign (Fig. 3). We could not determine malignancy risk for hypovascular nodules detected on TRMRA, from our limited number of cases with hypovascular nodules. Given the fact that we do not know the risk of malignancy for hypovascular nodules identified by TRMRA, it would be reasonable to perform follow-up US for hypovascular nodules detected on TRMRA in consideration of the patients' risk factors.
As only one pathologically proven malignant nodule was found in our study, we could not determine any specific features on TRMRA distinguishing between benign and malignant thyroid nodules. In our study, both benign and malignant nodules appeared to have a similar pattern regarding a time-signal intensity curve. In a way, TRMRA is analogous to dynamic contrast enhanced (DCE) MR imaging, as the acquisition of the same slices at multiple time points can be achieved using both techniques in such a way that time-signal intensity curves can be obtained for a specific ROI. Recent studies have documented that the enhancement time-intensity curve of a thyroid nodule on DCE MRI is related to its histopathologic features (19, 20). These authors suggested that the delayed washout pattern of the contrast enhancement was especially relevant for thyroid carcinomas. TRMRA could also be used in the evaluation of enhancement kinetics similar to DCE MRI. Hence, we suggest that a large-scale investigation is required to determine whether there is a difference in the enhancement kinetics of benign and malignant thyroid nodules.
In our study, of the 30 incidentalomas (80%) found in the 26 patients who underwent subsequent thyroid US, 3 nodules in 3 patients were not identified by US. We postulate that the causes of this are the small size, isoechogenecity or peripheral location of the incidentalomas. In addition, all the 3 nodules were not notified on the initial radiologic report of TRMRA. Hence, thyroid US should have been taken for screening without an awareness of the presence of the incidentaloma on TRMRA.
A few limitations to our study should be addressed; first, only a relatively small number of TRMRA cases could be further examined by using US and cytopathology. This was mainly due to the retrospective nature of our study. Second, most of the diagnoses were made by cytology rather than by histology. A well-designed prospective study including consecutive thyroid aspiration biopsies will be needed for further investigation of the true clinical significance of incidental thyroid nodules found on TRMRA.
In conclusion, we found thyroid incidentalomas to be present in 4.5% (102/2010) of thyroid incidentalomas on TRMRA examinations. The frequency of malignancy in these lesions was not higher than that of other imaging modalities. Thyroid incidentalomas on TRMRA did not necessarily indicate malignancy. However, when encountering such a lesion in clinical practice, a subsequent thyroid US is certainly warranted.
Figures and Tables
Fig. 1
58-year-old man with left thyroid nodule, subsequently diagnosed as benign by US-guided FNAB.
A. Coronal MIP image of TRMRA shows left thyroid nodule with homogenous hypervascularity. B. Transverse US image of left thyroid reveals smooth, oval, isoechoic, solid nodule, classified as indeterminate. FNAB = fine needle aspiration biopsy, MIP = maximum intensity projections, TRMRA = time-resolved MR angiography, US = ultrasonographic
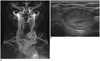
Fig. 2
60-year-old man with left thyroid nodule, subsequently diagnosed as benign by US-guided FNAB.
A. Coronal MIP image of TRMRA shows left thyroid nodule with inhomogenous hypervascularity (black lined arrows). B. Longitudinal US image of left thyroid reveals smooth, oval, isoechoic predominantly cystic nodule, classified as indeterminate. Internal hypervascular areas (black lined arrows on A) correlate with isoechoic solid portion (arrows). MIP = maximum intensity projections, TRMRA = time-resolved MR angiography, US = ultrasonographic
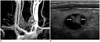
Fig. 3
66-year-old man with right thyroid nodule.
A. Coronal MIP image of TRMRA shows right thyroid nodule (black lined arrows) with hypovascularity. B. Longitudinal US image of right thyroid reveals smooth, oval, isoechoic, predominantly cystic nodule with comet tail artifacts (arrows), which is probably classified as benign nodule. MIP = maximum intensity projections, TRMRA = time-resolved MR angiography, US = ultrasonographic
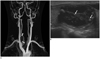
Fig. 4
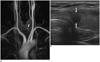
49-year-old woman with left papillary thyroid carcinoma.
A. Coronal MIP image of TRMRA shows left thyroid nodule (black lined arrow) with homogenous hypovascularity. B. Transverse US image of left thyroid shows spiculated, round, hypoechoic, solid nodule, classified as suspicious. MIP = maximum intensity projections, TRMRA = time-resolved MR angiography, US = ultrasonographic
References
1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
2. Lohan DG, Barkhordarian F, Saleh R, Krishnam M, Salamon N, Ruehm SG, et al. MR angiography at 3 T for assessment of the external carotid artery system. AJR Am J Roentgenol. 2007. 189:1088–1094.
3. Markl M, Uhl M, Wieben O, Ness T, Langer M, Hennig J, et al. High resolution 3T MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging. 2006. 24:423–427.
4. Nael K, Michaely HJ, Villablanca P, Salamon N, Laub G, Finn JP. Time-resolved contrast enhanced magnetic resonance angiography of the head and neck at 3.0 tesla: initial results. Invest Radiol. 2006. 41:116–112.
5. Lohan DG, Tomasian A, Saleh R, Krishnam M, Finn JP. Hypervascular thyroid nodules on time-resolved MR angiography at 3 T: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008. 190:W255–W260.
6. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.
7. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011. 12:1–14.
8. Youserm DM, Huang T, Loevner LA, Langlotz CP. Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol. 1997. 18:1423–1428.
9. Yoon DY, Chang SK, Choi CS, Yun EJ, Seo YL, Nam ES, et al. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr. 2008. 32:810–815.
10. Iyer NG, Shaha AR, Silver CE, Devaney KO, Rinaldo A, Pellitteri PK, et al. Thyroid incidentalomas: to treat or not to treat. Eur Arch Otorhinolaryngol. 2010. 267:1019–1026.
11. Ahmed S, Horton KM, Jeffrey RB Jr, Sheth S, Fishman EK. Incidental thyroid nodules on chest CT: Review of the literature and management suggestions. AJR Am J Roentgenol. 2010. 195:1066–1071.
12. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003. 22:1083–1090.
13. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 87:1941–1946.
14. Holden A. The role of colour and duplex Doppler ultrasound in the assessment of thyroid nodules. Australas Radiol. 1995. 39:343–349.
15. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003. 22:127–131. quiz 132-134.
16. Pacella CM, Guglielmi R, Fabbrini R, Bianchini A, Rinaldi R, Panunzi C, et al. Papillary carcinoma in small hypoechoic thyroid nodules: predictive value of echo color Doppler evaluation. Preliminary results. J Exp Clin Cancer Res. 1998. 17:127–112.
17. Iared W, Shigueoka DC, Cristófoli JC, Andriolo R, Atallah AN, Ajzen SA, et al. Use of color Doppler ultrasonography for the prediction of malignancy in follicular thyroid neoplasms: systematic review and meta-analysis. J Ultrasound Med. 2010. 29:419–425.
18. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010. 255:260–269.
19. Kusunoki T, Murata K, Nishida S, Tomura T, Inoue M. Histopathological findings of human thyroid tumors and dynamic MRI. Auris Nasus Larynx. 2002. 29:357–360.
20. Tezelman S, Giles Y, Tunca F, Gok K, Poyanli A, Salmaslioglu A, et al. Diagnostic value of dynamic contrast medium enhanced magnetic resonance imaging in preoperative detection of thyroid carcinoma. Arch Surg. 2007. 142:1036–1041.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download