Abstract
Objective
This study used functional magnetic resonance imaging (fMRI) to contrast the differential brain activation patterns in response to visual stimulation with both male and female erotic nude pictures in male-to-female (MTF) transsexuals who underwent a sex reassignment surgery.
Materials and Methods
A total of nine healthy MTF transsexuals after a sex reassignment surgery underwent fMRI on a 3.0 Tesla MR Scanner. The brain activation patterns were induced by visual stimulation with both male and female erotic nude pictures.
Results
The sex hormone levels of the postoperative MTF transsexuals were in the normal range of healthy heterosexual females. The brain areas, which were activated by viewing male nude pictures when compared with viewing female nude pictures, included predominantly the cerebellum, hippocampus, putamen, anterior cingulate gyrus, head of caudate nucleus, amygdala, midbrain, thalamus, insula, and body of caudate nucleus. On the other hand, brain activation induced by viewing female nude pictures was predominantly observed in the hypothalamus and the septal area.
Transsexualism is a type of gender identity disorder and is defined as the desire to live and be accepted as a member of the opposite sex relative to the genetic sex. The desire for being the opposite sex is accompanied by a persistent discomfort with one's natal body and a wish to 264have any hormonal and/or surgical therapy to make one's body as similar as possible to the preferred sex (1, 2). Male-to-female (MTF) transsexual individuals are defined as those transgenders who have had a MTF sex reassignment surgery with castration of the penis and testicles to alter their bodies to be similar to the opposite sex (3, 4). Transsexualism is a very rare phenomenon, and its prevalence differs in each country. Although there are no reliable statistics regarding the prevalence of transgenders in Korea, there are approximately 1400 transgenders nationwide (5).
In the past decade, numerous neuroimaging studies (6-18) using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have been performed to reveal the brain centers associated with sexual arousal. In particular, the fMRI is being widely used in such studies because of its advantages over PET, noninvasiveness, and high spatiotemporal resolution (19, 20). Most studies that have been performed up to now dealt with normal persons or patients with sexual disorder; however, studies for the transsexuals with a sex reassignment surgery have not yet been reported. Although a single study (18) has been carried out to define differential cerebral activation patterns in response to visual erotic stimuli in MTF transsexuals, the subjects recruited were MTF transsexuals without any sex reassignment surgery, and sex hormone levels of the subjects were not considered in conjunction with brain activation. Recently, Gizewski et al. (18) reported that significantly different brain activated areas including the left thalamus, bilateral amygdala, orbitofrontal, and insular cortex were observed as a result of the visual erotic stimuli in MTF transsexuals when compared with male controls; however, no such significant difference in activation patterns were observed when compared with female controls. Their findings suggested that MTF transsexuals have a specific feature of a female-like brain function.
Although a sex reassignment surgery does not affect the sexual orientation, the postoperative MTF transsexuals would be more satisfied with their gender identity and sexual behavior than ever, resulting in a more reliable emotional response to erotic visual stimuli. We used 3.0 Tesla fMRI to study the differential brain activation patterns evoked by viewing a string of male and female nude pictures, and further to clarify the gender identity of the postoperative MTF transsexuals in terms of functional neuroanatomy associated with sexual arousal.
Nine sex-reassigned MTF transsexuals (age 25-47 years; mean age: 39.7 ± 6.3), who underwent sex reassignment surgery, including castration of testicles and reformation of a neovagina using the inverted penile skin and a mammoplasty, were recruited through a questionnaire and an interview by a psychiatrist. All subjects were homosexual and right-handed subjects with no history of neurological or psychiatric illness. The subjects had undergone sex reassignment surgery 2-15 years prior to this study, and have been on hormone supplementary therapy since the sex reassignment surgery. They exhibited a passion for female traits such as female clothes, accessories, and long hair. In addition, their voice was husky, but with a feminine way of talking. All subjects gave a fully informed consent and experimental contents in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board.
The levels of serum sex hormones such as free testosterone (Free T), dehydroepiandrosteronesulfate (DHEA-S), sex hormone-binding globulin (SHBG), and estriol (E3) were measured by radioimmunoassay (RIA), while the levels of hormones such as estradiol (E2), follicle stimulating hormone (FSH), and luteinizing hormone (LH) were measured by chemiluminescent immunoassay (CLIA). The average levels of Free T, DHEA-S, SHBG, E2, FSH, and LH in MTF transsexuals were 0.16 ± 0.14 pg/mL, 136.90 ± 63.54 µg/dL, 59.22 ± 28.45 nmol/L, 16.78 ± 8.09 pg/mL, 36.87 ± 16.41 mLU/mL, and 12.82 ± 5.01 mLU/mL, respectively. The levels of Free T, DHEA-S, and SHBG were in the normal range of Korean women, while those of E2 and FSH were in the normal range of postmenopausal women. The level of LH was in the ranges of the luteal and/or midcycle of premenopausal women (21, 22).
All images were obtained using a 3.0T Magnetom Trio MR Scanner (Siemens Medical Solutions, Germany) with a birdcage head coil. The high-resolution T1-weighted images (repetition time [TR]/echo time [TE] = 400 ms/8 ms) and T2-weighted images (TR/TE = 4000 ms/88 ms) were obtained with the following parameters: field of view (FOV) = 22 cm × 22 cm, matrix size = 192 × 192, number of excitations (NEX) = 2, and slice thickness = 5 mm.
Functional images were acquired from a gradient-echo echo-planar pulse sequence with the following parameters: TR/TE = 3000 ms/30 ms, flip angle = 90°, FOV = 22 cm × 22 cm, matrix size = 64 × 64, NEX = 1, and slice thickness = 4 mm. The fMRI data were obtained from the 25 slices parallel to the AC-PC (anterior commissure and posterior commissure) line on the transverse plane, giving a total of 3000 images. The first two phases of dummy scans were supplemented to circumvent unstable fMRI signals.
The paradigm for visual sexual stimulation consisted of two alternating periods of rest and activation, and it began with 1 minute rest, 90 second stimulation with a string of erotic female nude pictures-1 minute rest-90 second stimulation with a string of erotic male nude pictures, and concluded with 1 minute rest. The time duration for viewing a single male or female erotic nude picture was 3 seconds. Every male or female erotic nude picture used for activation of the subject's brain was edited and approved by a urologist and a psychologist, who had majored in sexual medicine. The pictures were not disclosed in advance in order to increase the sexual arousal of the subjects. The visual stimuli were generated on a computer and projected via a liquid crystal display projector onto a screen located on the head coil in front of the subject's forehead.
The fMRI data were analyzed by post-processing and data analysis using Statistical Parametric Mapping (SPM02, Wellcome Department of Cognitive Neurology, University College London, London, UK). The initial images were realigned to correct the head movement and were subsequently reconstructed to a volume image. The volume image was spatially normalized to the Montreal Neurological Institute (MNI) space. Normalized images were then smoothed with a spatial Gaussian filter with an 8-mm full-width-at-half-maximum (FWHM). After following the specification of the appropriate design matrix, signal changes in the hemodynamic response produced by the different experimental conditions were assessed at each voxel using a general linear model (GLM). Significant signal changes for each contrast were assessed by independent t test on a voxel-by-voxel basis.
For the group analysis, these individual contrast images were entered into an independent two sample t test to compare the differential brain activation patterns in response to visual stimulation with male and female erotic nude pictures. The threshold of the t-statistic was set to p < 0.05. Reported coordinates identify the voxel with peak activity within the cluster of activation. Based on the fMRI and PET studies (6-18), which had been published, we identified 12 brain regions of interest (ROIs) associated with visual sexual arousal and these ROIs were potentially chosen to be the key brain center for this study (Table 1).
Figure 1 compares the differential activation patterns in response to visual stimulation with male and female erotic nude pictures, which were analyzed by two sample t test and their brain activities were summarized in the order of increasing t value in Table 2. The areas observed to have predominant activation on viewing the male erotic nude pictures as compared with female pictures included the cerebellum, hippocampus, putamen, anterior cingulate gyrus, head of caudate nucleus, amygdala, midbrain, thalamus, insula, and body of caudate nucleus (Fig. 1A, Table 2). On the other hand, the areas that showed predominant brain activation on viewing the female nude pictures were observed in the hypothalamus and the septal area (Fig. 1B, Table 2).
It has been proposed that human sexual arousal is a multidimensional experience comprising four closely interrelated and coordinated components: cognitive, emotional, motivational, and physiological (6, 7, 14). The cognitive component involves an initial process of appraisal for sexual stimulus, and the emotional component refers to the specific hedonic quality of sexual arousal. The motivational component relates to direct behavior to a sexual goal and the physiological component regards the autonomic and endocrinological responses associated with sexual arousal (6, 7, 14). As for the physiological component, the levels of sex hormones are particularly important in conjunction with the sexual function and behavior associated with sexual orientation (23). Administration of cross-sex hormones to transsexuals induces distinct changes in brain morphology as well as sexual behavior and cognition (23). The physical changes induced by estrogen hormone therapy in MTF transsexuals involve increased breast hemi-circumference and more breast development in proportion to the hormone volume, regardless of the dosage of estrogen (24). In addition, although no changes in penis length were observed in patients receiving female hormones, testicular volume was reduced by approximately 50%. Pol et al. (23) have assessed the changes in the total brain volume and the hypothalamus volume in young adult transsexuals with cross-sex hormone treatment. They revealed that with administration of estrogens and anti-androgens, the brain size decreased by 31 mL over a four-month period, together with changes in sexual behavior and cognition. In this study, we assumed that morphological changes in the brain of transsexuals are accompanied by neuro-functional changes. In order to clarify our assumption, a noninvasive neuroimaging technique such as fMRI was recommended, which also helped to evaluate the differential brain activation patterns associated with sexual arousal.
Recently, several lines of evidence have been published to investigate brain activation areas in response to visual sexual stimuli in patients with sexual disorder. Safron et al. (15) distinguished the brain areas associated with preferred and nonpreferred visual sexual stimuli between homosexual and heterosexual men using an fMRI. They suggested that both homosexual and heterosexual men exhibited category-specific arousal in brain activity, and demonstrated widespread increase in evoked activity for preferred stimuli. In a similar study (16), both heterosexual and homosexual groups showed no activation of the hypothalamus when viewing videos of their opposite sexual orientation. The brain areas showing significant activity in MTF subjects without any sex reassignment surgery and hormone therapy compared with male controls included the left thalamus, bilateral amygdala, orbitofrontal, and insular cortices (18). In our study, sexual orientation of MTF transsexuals was assessed from the brain activation patterns in response to visual sexual stimulation on the assumption that differential brain activation patterns resulting from visual sexual stimulation with two different strings of male or female erotic nude pictures should be indicative of their sexual orientation.
Note that the average levels of sex hormones including Free T, E2, and FSH in the postoperative MTF transsexuals who participated in our study are within the normal range of Korean women (21), and their physical and mental states were very similar to a biological female with heterosexual orientation. The differential brain areas showing predominant activation, while viewing the male nude pictures as compared to female pictures, included the cerebellum, hippocampus, putamen, anterior cingulate gyrus, head of caudate nucleus, amygdala, midbrain, thalamus, insular, and body of caudate nucleus. On the other hand, the predominant brain activation induced by viewing female nude pictures was observed in the hypothalamus and the septal area. It has been proposed that the thalamic nuclei are a part of a neural network comprising the amygdala and orbitofrontal cortex in primates. This putative network is potentially involved in emotional processing (25). This extensive thalamocortical interconnectivity has been theorized to constitute a neuronal basis for conscious awareness (26, 27), and therefore, the thalamus might be related to the cognitive dimension of sexual arousal. In light of the anatomic projections that the amygdala sends to the hypothalamus and temporal pole (28), it appears conceivable that in the sexual arousal condition, the right amygdala, right hypothalamus, and right anterior temporal pole are functional components of a neural circuit subserving the processing of visual erotic stimuli (29). According to Hamann et al. (11), the amygdala is more strongly activated in males than in females, while viewing identical sexual stimuli. The hippocampus processes intellectual, emotional, and actual information through the memory and experience, and is involved in a specific role with the amygdala during sexual arousal (15, 30). Activation of the hippocampus and amygdala noted here may thus be related to the appraisal process through which the erotic stimuli depicted in the male erotic nude pictures were evaluated as a sexual incentive (26).
There is evidence that activation of the anterior cingulate gyrus (ACG) is correlated to emotion, attention, sexual motivation, and possibly, the maintenance of correspondence between the sexual response and the affective value of the stimulus (7, 12). The ACG is a part of the limbic system and plays a critical role in initiation, motivation, and goal-directed behaviors. More specifically, in animals, stimulation of the anterior cingulate gyrus has an effect on autonomic and endocrine functions, including penile erection and gonadal hormone secretion (6, 31). This area also projects to the claustrum and putamen and, in our study, the putamen and caudate nucleus were activated by viewing the male erotic nude pictures only.
Another interesting finding in our study is the activation of the insula that is extensively interconnected with the amygdala. In particular, the anterior part of the insula participates in determining the affective state of experience and behavior (6, 32). Oppenheimer et al. (33) suggested that electrical stimulation in the anterior insula promotes autonomic responses in both human and monkeys. This finding is consistent with the fact that the insula is highly interconnected with regions involved in regulation of the autonomic nervous system (34). Therefore, the insular activation noted here might be a neural correlate of the autonomic changes associated with sexual arousal (26). The midbrain, another significant area in our study, showed predominant brain activation in male nude pictures. The midbrain is a relatively small region surrounded by CSF and contains a large proportion of white matter (8). Mapping of this area is difficult because brainstem motion is related to heartbeats (35). In general, the midbrain is a station between the hypothalamus and the peripheral and autonomic nervous systems. It transmits somatosensory information from the genitals to the medial preoptic nucleus via its reticular formation cells and connects the hypothalamus with autonomic centers via the periaqueductal gray matter (8). We also observed that activation of the cerebellum took place when viewing the male nude pictures only. The nerve fibers of the cerebellum transmit to the primary motor area of the cerebral cortex from the thalamus via the red nucleus. This area also links to the pons, medulla oblongata, and spinal cord via others nerve fibers. The cerebellum receives input from the proprioceptive receptor, and plays an important role in motor control together with the basal ganglia and motor area of the cerebral cortex (36). On the basis of the fact that activation of the cerebellum with the basal ganglia has often been noted in functional brain imaging studies on sexual arousal (29), we assume that the cerebellum is related to the "feeling" experience associated with sexual arousal, providing genital erection.
In our study, the predominant activation induced by viewing female erotic nude pictures over the male pictures was observed in the hypothalamus and septal area. Of all the brain areas involved in sexual arousal, the hypothalamus plays an important role in the regulation of sexual behavior in animals (37, 38). This area contains a number of small nuclei with a variety of functions, where salient sexual dimorphisms have been shown in various hypothalamic subregions (39). It has been proposed that such dimorphism, which may result from early influences of gonadal hormones, might somehow be linked to gender differences in sexual behavior (40, 41). It is also known that the connectivity patterns of relative synapse in the hypothalamus are in connection to gender difference (42). The lesions in the medial preoptic area of the hypothalamus, in particular, impair copulation behavior in males of various species (42). Activation of the hypothalamus noted in our study is not consistent with the results of previous studies (26, 43) in which positive correlation between sexual arousal and magnitude of hypothalamic activation in males was related to a high correlation between sexual arousal and physiological indices of sexual response such as penile tumescence (43). Another interesting brain action was observed in the septal area, which can hardly be observed in the fMRI system at low magnetic field strength. In the past years, all fMRI studies concerning visual sexual stimuli in MTF transsexuals have been performed on 1.5T MRI system (16, 18). Due to the dominant effect of large vessels with low magnetic field at 1.5 Tesla, it is hard to detect activation from such a small region, the septal area (44). The septal area has major anatomic connection to the frontal cortex, hypothalamus, and amygdaloid nuclei, and it is defined as an important brain structure mediating sexual behavior in human males and regulating masculine and feminine sexual behavior as well (45, 46). Although it is clear that activation of the hypothalamus and the septal area is closely related with sexual arousal, it is not clear why predominant activation of both the brain areas is observed while viewing female nude pictures opposite to the sexual orientation of the MTF transsexuals. However, it is important to note that differential brain activation on fMRI reflects differential sexual preference.
One of the limitations of our study was the small number of subjects, which is mainly due to the negative attitude toward people with sexual disorder in most oriental countries, providing limited statistical power to the data. Another limitation was that, the assessment of sexual arousal with visual stimuli was carried out by a self-report and an interview.
In this study, we demonstrated that distinct brain centers with predominant activity induced by viewing male erotic nude pictures over female pictures in postoperative MTF transsexuals with cross-sex hormone supplement reflect their sexual orientation opposite to the genetic sex. This study would be helpful to provide valuable information for the assessment of patients with gender identity disorders and for preclinical planning of a sex reassignment surgery as well.
Figures and Tables
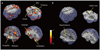 | Fig. 1
Differential brain activation patterns in response to visual stimulation with male and female erotic nude pictures, which were processed by two sample t test (p < 0.05), providing contrasts of "male nude pictures" over "female nude pictures" (A) and "female nude pictures" over "male nude pictures" (B). The three-dimensional images were generated using MRIcro (ver. 1.39) software. |
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 1994. 3rd ed. Washington, DC: American Psychiatric Association.
2. World Health Organization. International statistical classification of diseases and related health problems. 1992. 10th revision. Geneva: World Health Organization.
3. Michel A, Mormont C, Legros JJ. A psycho-endocrinological overview of transsexualism. Eur J Endocrinol. 2001. 145:365–376.
4. Swaab DF. Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol. 2004. 19:301–312.
5. Park JM, Kwon YS, Lee KC, Kim SK, Kwak H, Kim SB. Hormonal analysis of female transgender patients performed gender reassignment operation. J Korean Soc Plast Reconstr Surg. 2005. 32:699–705.
6. Stoléru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.
7. Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000. 11:162–177.
8. Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, et al. Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O-H2O PET study in heterosexual men. Neuroimage. 2001. 14(1 Pt 1):105–117.
9. Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001. 57:1189–1194.
10. Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002. 125(Pt 5):1014–1023.
11. Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004. 7:411–416.
12. Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005. 26:1086–1096.
13. Jeong GW, Park K, Youn G, Kang HK, Kim HJ, Seo JJ, et al. Assessment of cerebrocortical regions associated with sexual arousal in premenopausal and menopausal women by using BOLD-based functional MRI. J Sex Med. 2005. 2:645–651.
14. Kim SW, Sohn DW, Cho YH, Yang WS, Lee KU, Juh R, et al. Brain activation by visual erotic stimuli in healthy middle aged males. Int J Impot Res. 2006. 18:452–457.
15. Toledano R, Pfaus J. The Sexual Arousal and Desire Inventory (SADI): a multidimensional scale to assess subjective sexual arousal and desire. J Sex Med. 2006. 3:853–877.
16. Safron A, Barch B, Bailey JM, Gitelman DR, Parrish TB, Reber PJ. Neural correlates of sexual arousal in homosexual and heterosexual men. Behav Neurosci. 2007. 121:237–248.
17. Paul T, Schiffer B, Zwarg T, Krüger TH, Karama S, Schedlowski M, et al. Brain response to visual sexual stimuli in heterosexual and homosexual males. Hum Brain Mapp. 2008. 29:726–735.
18. Yang JC, Park K, Eun SJ, Lee MS, Yoon JS, Shin IS, et al. Assessment of cerebrocortical areas associated with sexual arousal in depressive women using functional MR imaging. J Sex Med. 2008. 5:602–609.
19. Gizewski ER, Krause E, Schlamann M, Happich F, Ladd ME, Forsting M, et al. Specific cerebral activation due to visual erotic stimuli in male-to-female transsexuals compared with male and female controls: an fMRI study. J Sex Med. 2009. 6:440–448.
20. Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990. 14:68–78.
21. Tsuchita H, Kuwata T. Trace lipid from whey-mineral complex enhances calcium availability in young ovariectomized rats. Br J Nutr. 1995. 73:299–309.
22. Green Cross Reference Lab. Sex hormones in green cross reference lab guidebook. 2007. Yongin, Korea: Green Cross Reference Lab;201–224.
23. Dove GA, Morley F, Batchelor A, Lunn SF. Oestrogenic function in postmenopausal women. J Reprod Fertil. 1971. 24:1–8.
24. Pol HEH, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, et al. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. 2006. 155:S107–S114.
25. Meyer WJ 3rd, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch Sex Behav. 1986. 15:121–138.
26. Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000. 52:319–330.
27. Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002. 16:1–13.
28. Llinás R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998. 353:1841–1849.
29. Martin JH. Neuroanatomy. 1996. Stanford: Appleton & Lange.
30. Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001. 21:RC165.
31. Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999. 2:289–293.
32. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995. 118(Pt 1):279–306.
33. Mesulam MM, Mufson EF. Peters A, Jones EG, editors. The insula of Reil in man and monkey. Architectonics, connectivity and function. 1985. 2nd ed. New York: Plenum Press;179–226.
34. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992. 42:1727–1732.
35. Cechetto DF. Identification of a cortical site for stress-induced cardiovascular dysfunction. Integr Physiol Behav Sci. 1994. 29:362–373.
36. Guimaraes AR, Melcher JR, Talavage TM, Baker JR, Ledden P, Rosen BR, et al. Imaging subcortical auditory activity in humans. Hum Brain Mapp. 1998. 6:33–41.
37. Fox SI. Human Physiology. 2009. New York: McGraw-Hill;226.
38. Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Knobil E, Neill JD, editors. Cellular and molecular mechanisms of female reproductive behaviors. Physiology of reproduction. 1994. New York: Raven Press;107–220.
39. Sachs BD, Meisel RL. Knobil E, Neill JD, editors. The physiology of male sexual behavior. Physiology of reproduction. 1994. New York: Raven Press;3–105.
40. Hofman MA, Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat. 1989. 164:55–72.
41. Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989. 9:497–506.
42. Hofman MA, Swaab DF. Sexual dimorphism of the human brain: myth and reality. Exp Clin Endocrinol. 1991. 98:161–170.
43. Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971. 173:731–733.
44. Ferris CF, Snowdon CT, King JA, Duong TQ, Ziegler TE, Ugurbil K, et al. Functional imaging of brain activity in conscious monkeys responding to sexually arousing cues. Neuroreport. 2001. 12:2231–2236.
45. Sakheim DK, Barlow DH, Beck JG, Abrahamson DJ. The effect of an increased awareness of erectile cues on sexual arousal. Behav Res Ther. 1984. 22:151–158.
46. Gorman DG, Cummings JL. Hypersexuality following septal injury. Arch Neurol. 1992. 49:308–310.
47. Kondo Y, Shinoda A, Yamanouchi K, Arai Y. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm Behav. 1990. 24:421–434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download