Abstract
Primary pancreatic hydatid cysts are rare and its percutaneous treatment and catheterization technique has, to the best of our knowledge, not been published in literature. A 33-year-old male patient who presented with abdominal pain was evaluated by ultrasonography (US) and computed tomography examinations. Both examinations revealed a cyst in the neck of the pancreas. After the administration of albendazole chemoprophylaxis, the patient underwent diagnostic puncture showing high pressure spring water which harbored the scoleces and was treated percutaneously by the catheterization technique. In this technique, first the cyst was punctured, the fluid content aspirated, the radiocontrast material injected to see possible fistulisation, and then re-aspirated. The 20% hypertonic saline solution was injected and re-aspiration was performed to the best of our abilities, followed by the insertion of a catheter for drainage of the remaining non-aspiratable fluid content. At follow-up examination, the cyst was not visible on US after 6 months. There was no evidence of cyst recurrence or dissemination after 18 months at serologic and imaging follow-up.
In endemic areas, hydatid disease is a serious health issue. Primary hydatid disease of the pancreas is extremely rare, accounting for 0.2% to 2% of all hydatid disease (1-3). Most of the pancreatic hydatid cysts reported to date have been treated surgically with significant morbidity (2). Percutaneous drainage (PD) is reported to be a promising treatment of choice in hepatic and pancreatic hydatid cysts (4-8). PD is performed either by puncture, aspiration of cyst content, injection of hypertonic saline solution, and re-aspiration of all fluid (PAIR) or by a catheterization technique based on the size of the cyst (7). Only one patient with a pancreatic hydatid cyst so far, was treated by the PAIR technique, but catheter drainage has not been used (4). In this report, we present a 33-year-old patient with a pancreatic hydatid cyst that was successfully treated by percutaneous catheter placement.
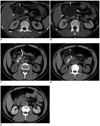
A 32-year-old male had abdominal pain for two months. The pain was located in the epigastric region, but not associated with any other symptoms such as nausea, vomiting, diarrhea, jaundice, or fever. The patient's physical examination was unremarkable. An ultrasonography (US) examination was performed, which revealed a pancreatic cystic mass at the neck of the pancreas (55 × 44 × 45 mm). A computed tomography (CT) scan of the upper abdomen did not show any other organ involvement, but a cystic pancreatic mass without any enhancement or calcification was identified (Fig. 1A, B). The differential diagnosis included pancreatic serous cystadenoma, pseudocyst, primary hydatid cyst of the pancreas, or cystadenocarcinoma. Serologic tests were positive for hydatid disease. PD was planned and informed consent was obtained. PD of the cyst was performed following 12 days of prophylactic oral albendazole treatment (10 mg/kg/day).
First the cyst was punctured with a 22 cm 22 gauge (G) cholangiography needle (Cook Europe, Bjaeverskov, Denmark) under US guidance using a 3.5 MHz convex probe. Next, the high pressure spring water-like cyst content was rapidly aspirated. A sample from this aspiration was sent for serologic and further microbiological evaluation. An immediate cytopathological exam reported the existence of scoleces in the aspirated cyst fluid, thus confirming hydatid disease. The cyst cavity was filled with 50 mL of 50% diluted sterile radiocontrast agents (iohexol 300 mgI/mL). The cyst did not show any sign of fistulisation to surrounding structures under fluoroscopy. Then, the radiocontrast filled cyst cavity was re-aspirated and hypertonic saline (20%) was injected to the cavity under fluoroscopy guidance, which was left there for 20 minutes. Following re-aspiration of the cyst cavity, a 10-French (F) pigtail catheter was placed into the cavity for 24 hr of gravity drainage. A CT scan was obtained to confirm the catheter position (Fig. 1C, D). After a 20-minute waiting period, the cyst was again completely aspirated and the cavity was irrigated with sterile saline (0.9% NaCl). During the procedure, endocyst separation from the pericyst was visualized as echogenic structures under real time US.
The catheter in the pancreatic cyst cavity was withdrawn after 24 hours. No peri-procedural life-threatening complication was encountered and the patient was discharged from the hospital on the 3rd day after the procedure. Albendazole was continued for 2 more months and the patient was followed up with monthly US examinations. No cyst was visible on US and CT examination over the 18-month follow-up period (Fig. 1E). Furthermore, no recurrence or evidence of dissemination in the peritoneum and solid abdominal organs was seen. The patient became symptom-free and the serology for hydatid disease returned negative.
Hydatid disease is an endemic problem in many regions such as the Mediterranean countries, Australia, New Zealand, South America, South East and Far East Asia, and the Middle Eastern countries (4, 9). Primary pancreatic involvement is very rare, accounting for 0.2% to 2% of all hydatid disease cases (1, 2). Cyst locations included the head (57%), corpus (24%), or tail (19%) of the pancreas (3, 10, 11). Cysts in the corpus or tail usually do not cause any symptoms. In our case, the cyst was located in the neck of the pancreas and the patient experienced only abdominal pain.
The diagnosis may be based on US, CT or magnetic resonance imaging (12). There are two classification systems [Gharbi and WHO classification (13)] used by radiologists and clinicians for evaluating hydatid cysts (Table 1). In our case, there were no calcification or hyperechoic bands corresponding to a daughter cyst. However the fact that our country is an endemic area for the disease made us suspect a hydatid disease. Our preliminary diagnosis was confirmed with serologic tests.
The conventional treatment for any abdominal organ involvement of large hydatid cysts is surgery. Faraj et al. (14) reported a case treated with laparoscopic surgery that offers minimal invasive surgery. However, surgery may cause haemorrhage, and the location of the cyst near major biliary or vascular structures may prevent a complete pericystectomy (4). In some patients, surgery is not recommended (elderly, surgical high risk) and has an attendant risk of mortality, significant morbidity, and prolonged hospital stay (15). Percutaneous drainage (PD) is reported to be a promising treatment of choice in hepatic hydatid cysts (4-8). PD is a good alternative to surgery and it can be combined with medical chemoprophylaxis using albendazole (4). In our case, we took the risk of peritoneal fluid spillage by approaching the cyst through a direct peritoneal route like Yattoo et al. (4) did . We however did not have the option to take the transhepatic approach. However, the utilization of fine needles and catheters as well as the advancements in US and CT techniques make the chance of spillage extremely unlikely (16). To reduce the risk of spillage and peritoneal dissemination, a combination of PD with medical chemoprophylaxis using praziquantel or albendazol before and after PD can be used (7, 16-18). We started albendazole treatment 12 days before the intervention and continued for 3 weeks. Our case had no complication during and after the procedure. Yattoo et al. (4) is the only report in literature for percutaneous management of pancreatic hydatid disease using the PAIR technique. However our case is different from theirs in that we used an additional 10 F pigtail catheter placement and left it for 24 hours to drain. Our technique of percutaneous treatment turned out to be successful, based on follow-up imaging studies, serological tests and patient symptomatology over the course of 18 months, without evidence of recurrence or dissemination of the hydatid disease.
By utilizing the catheter technique, faster volume reduction and sclerosis of the cyst cavity walls is achieved at a rate compared to the simple PAIR technique since a significant amount of volume remains undrained in the cyst despite any effort following injection and drainage of the hypertonic saline in the PAIR technique. Re-aspiration of the entire cyst fluid content is usually not possible through the puncture needle after injection of hypertonic saline, as the germinative membrane layers detach, while multi-loculated fluid collection remain behind. For that reason it becomes a CE3 hydatid cyst according to the WHO classification (Table 1). Besides scoleces disintegrating into the new multi-loculated cyst-fluid end up, occlusion of the needle lumen occurred. Hence catheter injection and drainage through multiple side holes and a large lumen become necessary. The catheter technique is mainly used for the cyst hydatid located in the liver. The rationale for holding the catheter 24 hours is to completely drain the cyst content. We used the same technique as described in liver hydatid cysts (19).
However, multivesicular cysts harboring many daughter cysts could be problematic for PD. Our experience and report on this case is limited to PD of pancreatic hydatid disease, although the long term follow-up results for hepatic hydatid cysts showed that there was no persistence of secondary vesicles in multivesicular cysts (14, 16). Further studies are needed to confirm the safety and efficacy of PD in the management of complex hydatid cysts of the pancreas.
Hydatid disease in the pancreas should be included in the differential diagnoses of the cystic lesions of the pancreas, especially in endemic areas. PD and hypertonic saline or alcohol injection for the treatment of purely cystic hydatid disease of the pancreas can be used as an alternative to surgery. Catheter placement and drainage can also be used if possible and when required, based on cyst size.
Figures and Tables
Fig. 1
Pancreatic hydatid cyst treated by percutaneous catheter placement in 33-year-old male.
A, B. Contrast-enhanced axial computed tomography (CT) images show cystic mass (white arrows) arising from neck of pancreas. C, D. Axial CT images without contrast show 10 F pigtail catheter in cyst cavity. E. Contrast-enhanced axial CT image obtained 18 months after treatment shows no cystic cavity.
References
1. Ismail K, Haluk GI, Necati O. Surgical treatment of hydatid cysts of the pancreas. Int Surg. 1991. 76:185–188.
2. Kattan YB. Hydatid cysts in pancreas. Br Med J. 1975. 4:729–730.
3. Shah OJ, Robbani I, Zargar SA, Yattoo GN, Shah P, Ali S, et al. Hydatid cyst of the pancreas. An experience with six cases. JOP. 2010. 11:575–558.
4. Yattoo GN, Khuroo MS, Zargar SA, Bhat FA, Sofi BA. Case report: percutaneous drainage of the pancreatic head hydatid cyst with obstructive jaundice. J Gastroenterol Hepatol. 1999. 14:931–934.
5. Filice C, Pirola F, Brunetti E, Dughetti S, Strosselli M, Foglieni CS. A new therapeutic approach for hydatid liver cysts. Aspiration and alcohol injection under sonographic guidance. Gastroenterology. 1990. 98:1366–1368.
6. Khuroo MS, Zargar SA, Mahajan R. Echinococcus granulosus cysts in the liver: management with percutaneous drainage. Radiology. 1991. 180:141–145.
7. Bastid C, Azar C, Doyer M, Sahel J. Percutaneous treatment of hydatid cysts under sonographic guidance. Dig Dis Sci. 1994. 39:1576–1580.
8. Etlik O, Arslan H, Bay A, Sakarya ME, Harman M, Temizoz O, et al. Abdominal hydatid disease: long-term results of percutaneous treatment. Acta Radiol. 2004. 45:383–389.
9. Echenique-Elizondo M, Amondarain Arratíbel JA. Hydatid disease of the pancreas. JOP. 2004. 5:51–52.
10. Gayral F, Bouree P, Jourdanne P, Millat B, Labayle D. [Hydatid cyst of the pancreas. One case (author's transl)]. Nouv Presse Med. 1981. 10:3787–3788.
11. Tissot E, Peschaud R, Vignal J, Francillon J. Le kyste Hydatique du pancréas. Lyon Chir. 1975. 71:127–130.
12. Coskun T, Kama NA, Dener C, Gözalan U. Primary hydatid disease of the pancreas. Am J Gastroenterol. 1997. 92:899–900.
13. WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003. 85:253–261.
14. Faraj W, Selmo F, Khalifeh M, Jamali F. Laparoscopic resection of pancreatic hydatid disease. Surgery. 2006. 139:438–441.
15. Morris DL. Morris DL, editor. Surgical management of hepatic hydatid cyst. Hydatid Disease Current Medical and Surgical Management. 1992. 1st ed. Oxford: Butterworth-Heinemann;57–75.
16. Khuroo MS, Dar MY, Yattoo GN, Zargar SA, Javaid G, Khan BA, et al. Percutaneous drainage versus albendazole therapy in hepatic hydatidosis: a prospective, randomized study. Gastroenterology. 1993. 104:1452–1459.
17. Giorgio A, Tarantino L, Francica G, Mariniello N, Aloisio T, Soscia E, et al. Unilocular hydatid liver cysts: treatment with US-guided, double percutaneous aspiration and alcohol injection. Radiology. 1992. 184:705–710.
18. Khuroo MS, Wani NA, Javid G, Khan BA, Yattoo GN, Shah AH, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997. 337:881–887.
19. Ustünsöz B, Akhan O, Kamiloğlu MA, Somuncu I, Uğurel MS, Cetiner S. Percutaneous treatment of hydatid cysts of the liver: long-term results. AJR Am J Roentgenol. 1999. 172:91–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download