Abstract
We report successful outcomes after endovascular placement of a stent graft in a 74- and a 77-year-old men, both of whom had malignant superior vena cava syndrome caused by squamous cell carcinoma. In each patient, successful palliation of the malignant superior vena cava syndrome was achieved by placement of a stent graft. No procedure-related complications were observed. The patients were asymptomatic until their deaths, seven and 14 months after stent graft placement, respectively.
Superior vena cava (SVC) syndrome is a common complication of malignancy, particularly in lung cancer. It is evident in 4% of lung cancer patients at diagnosis (1), and it may also develop during the disease course. Patients with malignant SVCS are typically seriously ill, and further deterioration is likely, due to the presence of unresectable advanced malignant tumors. Although bypass surgery has been reported for palliative treatment of malignant SVCS in selected patients (2), this type of surgery in terminally ill patients is difficult to justify and rather invasive for a palliative procedure. Until recently, radiotherapy and chemotherapy were standards in the management of malignant SVCS (3, 4). However, both therapies may not be possible under certain conditions, especially when the cumulative maximum dosage has been reached in previous treatments. In addition, it may take several weeks before either intervention shows a clinical effect (5).
Endovascular stent placement as an alternative palliative treatment has increased because it is fastest way to relieve symptoms (6). Many studies have reported the efficacy of stent placement in patients with malignant SVCS (7-10). A few reports have described stent-graft placement in recurrent SVCS after bare metallic stent placement or iatrogenic injury of the SVC (11-14). However, initial placement of stent-grafts for the treatment of malignant SVCS has not been reported. Thus, we report on successful outcomes after the placement of stent-grafts for two patients who had malignant SVCS caused by squamous cell carcinoma.
A 74-year-old man presented with chest pain, anorexia, and dyspnea. A chest radiograph showed an ill-defined mass in the right upper lung field. Computed tomography (CT) revealed an ill-defined infiltrating central mass with mediastinal lymphadenopathy. The patient underwent a bronchoscopy and biopsy. Histology confirmed the diagnosis of squamous cell carcinoma and consequently, the patient underwent four cycles of chemotherapy for two months. The patient then presented with swelling of the face and severe dyspnea for a week. Subsequent CT demonstrated progression of the mass, obstruction of the SVC, confluence of both brachiocephalic veins (Fig. 1A), and right brachiocephalic venous thrombosis (Fig. 1B).
Although thrombolysis of the right brachiocephalic thrombosis and subsequent bilateral stenting could be performed, there was a risk of complications following thrombolysis because of the patient's advanced age. We thus decided on the unilateral placement of a triple-layered stent-graft (Vascular ComVi stent-graft, TaeWoong Medical, Gimpo, Korea) that was 5 mm long at both bare extensions (Fig. 1C). The triple-layered stent-graft was composed of two uncovered nitinol self-expanding metallic stents and an expanded polytetrafluoroethylene (ePTFE) membrane between two uncovered stent layers. The wire was exposed on both the inner and outer surfaces. The stent-graft was mounted onto an 8.5-Fr stent introducer set. After puncture of the right common femoral vein, a 9-Fr sheath was inserted. Left brachiocephalic venography demonstrated obstruction of the SVC and confluence (Fig. 1D). The pressure gradient between the left brachiocephalic vein and distal SVC was 24 mm Hg. Predilatation using a 10-mm-diameter balloon catheter (Boston Scientific, Galway, Ireland) was performed to determine the stent size and allow for the easy navigation of the stenosis as well as placement of the stent. Venography revealed that the length of the lesion was approximately 3 cm and the diameter of the SVC was approximately 12 mm. To prevent proximal and distal tumor overgrowth, a 14 mm × 8 cm stent-graft was introduced over a 0.035-inch, 180-cm-long extra stiff Amplatz guide-wire (Cook, Bloomington, IN, USA), which was deployed successfully across the stenosis (Fig. 1E). Post-stenting balloon dilation of the stent was then performed using a 10 mm × 4 cm balloon catheter. Venography immediately after stent placement confirmed the correct positioning of the stent and the pressure gradient decreased to 6 mm Hg. Clinical symptoms improved immediately after stent placement. The patient did not receive any prophylactic anticoagulation after stent placement. CT performed after 11 months demonstrated good stent patency without migration (Fig. 1F). The patient died at home 14 months after stent placement. At the time of death, the patient showed no recurrent symptoms of SVCS, suggesting continued patency of the stent.
A 77-year-old man presented with cough, whitish sputum, and dyspnea. A chest radiograph showed an ill-defined mass in the right upper lung field. CT revealed an ill-defined infiltrating central mass and a mediastinal lymphadenopathy. The patient underwent bronchoscopy and biopsy. Histology confirmed the diagnosis of squamous cell carcinoma and the patient underwent four cycles of chemotherapy for three months. The patient then presented with swelling of the face and severe dyspnea for 10 days. Subsequent CT demonstrated progression of the mass and obstruction of the SVC caused by the mass and mediastinal lymphadenopathy (Fig. 2A).
Right internal jugular venography via the right common femoral vein demonstrated obstruction of the SVC with venous collateral development (Fig. 2B). The pressure gradient between the right internal jugular vein and distal SVC was 21 mm Hg. Venography revealed that the length of the lesion was approximately 4.5 cm and the diameter of the SVC was approximately 11 mm. A 14 mm × 8 cm stent-graft (Fig. 2C) was successfully inserted. To prevent brachiocephalic vein occlusion by the stent-graft, the distal end of the stent-graft was deployed just beneath the confluence. Venography immediately after stent placement confirmed the correct positioning of the stent and the pressure gradient decreased to 7 mm Hg. Clinical symptoms improved immediately after stent placement and did not receive any prophylactic anticoagulation. CT performed after four months demonstrated good stent patency without migration (Fig. 2D). The patient died at home seven months after stent placement. At the time of death, the patient showed no recurrent symptoms of SVCS, suggesting continued patency of the stent.
Endovascular stenting may be used as a first-line therapeutic measure in patients with malignant SVCS because stenting does not interfere with subsequent antitumor treatments and provides urgent relief of symptoms (6, 8). Additionally, endovascular stenting greatly improves the quality of life of patients, who usually have a short life expectancy (8). Although many studies have reported the efficacy of endovascular stenting in patients with malignant SVCS, recurrence following initial successful stenting still occurs in up to 18% of cases (10), and is attributed to tumor ingrowth inside the stent or venous thrombosis. However, most patients with malignant SVCS have a short life expectancy and the stent remains patent until death. Furthermore, stent occlusion can be treated with thrombolysis, balloon dilation, or further stent insertion with good secondary patency rates. However, secondary endovascular treatments are sometimes difficult and have additional costs. Anticoagulation therapy is often prescribed for patients with malignant SVCS after stenting, although its efficacy has not been clearly confirmed (8). Moreover, determining the status of optimum anticoagulant therapy is difficult (10). Thus, to prevent stent occlusion and improve the patency rate of stents, we thought that it was important to prevent tumor ingrowth into the stents. This had led to the study of stent-grafts that are effective in preventing tumor ingrowth and subsequent stent occlusion.
Endovascular stent-graft placement has been used for emergency repair of the SVC (13, 14). Another case involved repair of recurrent SVC obstruction that occurred immediately or three weeks after metallic stent placement (11, 12). To our knowledge, in contrast to previously reported cases, our cases are the first to include the primary placement of an ePTFE-covered stent in patients with malignant SVCS.
Prior to using a stent-graft, a few technical points deserve special mention. First, risk of stent migration exists: stent migration remains a significant long term complication and is occasionally fatal (15). Stent migration occurred in neither of our cases because the partially covered stent with both bare extensions and outer bare stent design might have prevented stent migration. Additionally, selection of a slightly oversized stent might also prevent migration. Generally, to avoid stent migration, the diameter of the stent should be 10-20% greater than that of the lesion (7). Second, placing a long length graft at the confluence of the brachiocephalic veins carries the risk of occlusion of the contralateral branch. This could potentially lead to thrombosis of the upper extremity veins. However, when both brachiocephalic veins were invaded by the tumor, unilateral brachiocephalic vein revascularization was sufficient and provided higher flow through the graft than when both brachiocephalic veins were revascularized (16). Nagata et al. (7) suggested that unilateral relief of obstruction may allow collateral flow from the contralateral vein via the cervical and intracranial route. The Case 1 patient, in whom the stent-graft was placed from the SVC to the left brachiocephalic vein, experienced symptomatic relief. Follow-up CT images confirmed patency of the stent-graft and contralateral veins via collateral flow of the cervical route.
In conclusion, the two study cases demonstrate the potential efficacy of stent-graft placement for the palliative treatment of malignant SVCS. Stent-graft placement appears to be a safe and effective method. Both patients were asymptomatic until their deaths 7 and 14 months after, respectively. However, further investigation is necessary because the patency of the stent-graft in the venous system remains to be determined.
Figures and Tables
Fig. 1
Seventyfour-year-old man with malignant superior vena cava syndrome due to squamous cell carcinoma.
A. Contrast-enhanced axial CT image shows central mass with mediastinal lymphadenopathy and left brachiocephalic venous obstruction (arrow). B. Axial CT image at upper level of A shows right brachiocephalic venous thrombosis (arrow). C. Partially expanded polytetrafluoroethylene-covered stent-graft used in these cases. D. Left brachiocephalic venography shows obstruction of confluence and proximal superior vena cava (arrow). E. Venography after stent-graft placement (14 mm × 8 cm) (arrowheads) shows fluent passage of contrast medium via stent. F. Contrast enhanced axial CT image obtained 11 months after stent-graft placement shows patent stent-graft.
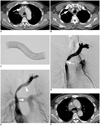
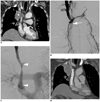
Fig. 2
Seventyseven-year-old man with malignant superior vena cava syndrome due to squamous cell carcinoma.
A. Contrast-enhanced coronal CT image shows superior vena cava obstruction caused by central lung mass (asterisk). B. Right brachiocephalic venography shows obstruction of proximal superior vena cava (arrow) with collaterals. C. Venography after stent-graft placement (14 mm × 8 cm) (arrowheads) shows fluent passage of contrast medium via stent. D. Contrast-enhanced coronal CT image obtained four months after stent-graft placement shows patent stent-graft.
References
1. Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol). 2002. 14:338–351.
2. Doty DB, Doty JR, Jones KW. Bypass of superior vena cava. Fifteen years' experience with spiral vein graft for obstruction of superior vena cava caused by benign disease. J Thorac Cardiovasc Surg. 1990. 99:889–895. discussion 895-896.
3. Armstrong BA, Perez CA, Simpson JR, Hederman MA. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys. 1987. 13:531–539.
4. Yellin A, Mandel M, Rechavi G, Neuman Y, Ramot B, Lieberman Y. Superior vena cava syndrome associated with lymphoma. Am J Dis Child. 1992. 146:1060–1063.
5. Dyet JF, Nicholson AA, Cook AM. The use of the Wallstent endovascular prosthesis in the treatment of malignant obstruction of the superior vena cava. Clin Radiol. 1993. 48:381–385.
6. Nicholson AA, Ettles DF, Arnold A, Greenstone M, Dyet JF. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997. 8:781–788.
7. Nagata T, Makutani S, Uchida H, Kichikawa K, Maeda M, Yoshioka T, et al. Follow-up results of 71 patients undergoing metallic stent placement for the treatment of a malignant obstruction of the superior vena cava. Cardiovasc Intervent Radiol. 2007. 30:959–967.
8. Lanciego C, Pangua C, Chacon JI, Velasco J, Boy RC, Viana A, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009. 193:549–558.
9. Cheng S. Superior vena cava syndrome: a contemporary review of a historic disease. Cardiol Rev. 2009. 17:16–23.
10. Nguyen NP, Borok TL, Welsh J, Vinh-Hung V. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax. 2009. 64:174–178.
11. Chin DH, Petersen BD, Timmermans H, Rosch J. Stent-graft in the management of superior vena cava syndrome. Cardiovasc Intervent Radiol. 1996. 19:302–304.
12. Gill K, Ettles DF, Nicholson AA. Recurrent superior vena caval obstruction due to invasion by malignant thymoma: treatment using a stent-graft. Br J Radiol. 2000. 73:1015–1017.
13. Azizzadeh A, Pham MT, Estrera AL, Coogan SM, Safi HJ. Endovascular repair of an iatrogenic superior vena caval injury: a case report. J Vasc Surg. 2007. 46:569–571.
14. Mansour M, Altenburg A, Haage P. Successful emergency stent implantation for superior vena cava perforation during malignant stenosis venoplasty. Cardiovasc Intervent Radiol. 2009. 32:1312–1316.
15. Martin M, Baumgartner I, Kolb M, Triller J, Dinkel HP. Fatal pericardial tamponade after Wallstent implantation for malignant superior vena cava syndrome. J Endovasc Ther. 2002. 9:680–684.
16. Dartevelle PG, Chapelier AR, Pastorino U, Corbi P, Lenot B, Cerrina J, et al. Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg. 1991. 102:259–265.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download