Abstract
Objective
Arsenic trioxide (As2O3) can be used as a possible pharmaceutical alternative that augments radiofrequency (RF) ablation by reducing tumor blood flow. The aim of this study was to assess the effect of intraarterial and intravenous administration of As2O3 on RF-induced ablation in an experimentally induced liver tumor.
Materials and Methods
VX2 carcinoma was grown in the livers of 30 rabbits. As2O3 (1 mg/kg) was administered through the hepatic artery (n = 10, group A) or ear vein (n = 10, group B), 30 minutes before RF ablation (125 mA ± 35; 90 ± 5℃). As a control group, 10 rabbits were treated with RF ablation alone (group C). RF was intentionally applied to the peripheral margin of the tumor so that ablation can cover the tumor and adjacent hepatic parenchyma. Ablation areas of the tumor and adjacent parenchymal changes among three groups were compared by the Kruskal-Wallis and Mann-Whitney U test.
Results
The overall ablation areas were 156 ± 28.9 mm2 (group A), 119 ± 31.7 (group B), and 92 ± 17.4 (group C, p < 0.04). The ablation area of the tumor was significantly larger in group A (73 ± 19.7 mm2) than both group B (50 ± 19.4, p = 0.02) and group C (28 ± 2.2, p < 0.01). The ratios of the tumoral ablation area to the overall ablation area were larger in group A (47 ± 10.5%) than that of the other groups (42 ± 7.3% in group B and 32 ± 5.6% in group C) (p < 0.03).
Radiofrequency (RF) ablation has been proved to be an effective treatment in unresectable malignant liver tumors (1-3). However, it has an inherent limitation in the volume of tumors which can be effectively ablated. Consequently, clinical data demonstrated excellent local tumor control (80-90%) in tumors < 3 cm in the diameter, but much less satisfactory results (50-75%) in larger tumors (1-3). This limitation is mainly caused by perfusion-mediated tissue cooling, heat sink effect (4). Therefore, several adjunctive techniques have been developed to reduce regional and intratumoral blood flow, and increase the ablation time. These include hepatic arterial occlusion by balloon catheter (5), transcatheter hepatic arterial embolization (6), bipolar array technique (7), saline injection (8), and pharmacologic modulation (9).
Contrary to their infamous carcinogenic properties as an environmental pollutant, arsenic compounds have been used for medical purposes for several thousand years (10). Recently, arsenic trioxide (As2O3) was found to be highly effective for the treatment of acute promyelocytic leukemia (APL) (11, 12). Thereafter, it has been widely tested in treating not only-APL leukemia but also a variety of solid tumors including hepatocellular carcinoma (HCC) (11, 13, 14). The mechanism of As2O3 activity against solid tumors has not been completely understood. However, previous studies discovered As2O3 induces tumor vascular shutdown, and subsequently potentiates hyperthermal therapy (15, 16). Hines-Peralta et al. (17) have shown that systematic administration of As2O3 enables larger zones of RF ablation in various experimentally induced tumor models. However, in their study, optimal results were achieved with a relatively high dose of this toxic drug and the synergistic effect has a narrow temporary window (18). Generally the hepatic artery is a better route of drug administration than systemic veins for the treatment of liver tumors (19, 20). Thus, we hypothesized that hepatic arterial administration of As2O3 may induce larger zones of RF ablation than intravenous administration. The purpose of this study is to assess the effects of hepatic arterial administration of As2O3 on RF-induced ablation and compare them with those of systemic venous administration in a liver tumor model.
All the experiments associated with this study were approved by our Institutional Animal Care And Use Committee. All rabbits were maintained according to the Guide for the Care and Use of Laboratory Animals (21). Thirty adult New Zealand white rabbits weighing 2.5-3.0 kg were used. The VX2 carcinoma strain had been maintained by means of successive transplantation into the hindlimb of the carrier rabbit. Anesthesia was induced with intravenous ketamine hydrochloride (50 mg per kilogram of body weight; Ketamine, Yuhan, Korea) and 2% xylazine (0.1 mL/kg; Rompun, Bayer, Germany). After a midline abdominal incision, 0.1 mL of minced VX2 carcinoma was implanted into the subcapsular parenchyma of the left medial lobe of the liver. Fourteen days after tumor implantation, when the tumors were anticipated to be 10-20 mm in diameter, the rabbits were used for the experiments.
One day before RF ablation, contrast-enhanced liver CT (Sensation 16; Siemens, Erlangen, Germany) was performed to confirm the presence and size of the liver tumors. On the basis of the longest diameter of the tumor, the rabbits were divided into three groups (groups A-C). The spontaneous necrosis of a VX2 liver tumor can be influenced by tumor size, which subsequently affects the results of our study. Therefore, rabbits with similar sized tumors were assigned to each group. The longest diameter of tumors ranged from 13.2 to 20.5 mm (18 ± 2.0 mm; median 17.4 mm mean ± standard deviation [SD]) in group A, 13.8 to 21.3 mm (17 ± 2.0 mm, 16.2 mm) in group B, and 11.7 to 20.0 mm (17 ± 3.1 mm, 17.6 mm) in group C. Tumor volumes were not statistically different among the three groups (p = 0.687, Kruskal-Wallace test).
A solution of As2O3 (Sigma, St. Louis, MO, USA) was prepared by magnetic stirring for one day at room temperature in a glass bottle and then placed the bottle into boiling water for one hour. After the solution became crystal clear, it was sterilized using a 0.2 M bottle-top filter and stored in the dark at 4℃. Just before the administration, the stock solution was then diluted with phosphate-buffered saline to a concentration of 1 mg/mL.
In group A, the As2O3 solution was administered through the hepatic artery. Under general anesthesia, an incision was made to access the right femoral artery. The celiac angiogram was obtained to identify the hepatic arterial anatomy and the feeder artery of the tumor using a 3-Fr microcatheter (Cook, Bloomington, IN, USA). After positioning the catheter within the proper hepatic artery, 1 mL/kg of the As2O3 solution (1 mg/kg) was slowly injected at a rate of 180 mL/hr using an infusion pump. The catheter was then removed and the femoral artery was ligated. In group B, the As2O3 solution was administered into the auricular vein. The amount of As2O3 solution and infusion rate were the same in group B. In group C, the same volume of phosphate-buffered saline was infused into the auricular vein.
One hour after completing the As2O3 solution or phosphate-buffered saline injection, RF ablation of the liver tumor was performed. Under general anesthesia, a midline incision was made to expose the liver. A 500-kHz RF generator (Valley-Lab, Boulder, CO, USA) was used to apply conventional monopolar RF energy. An internally cooled, 17-gauge electrode (Radionics) with a 1-cm active tip was placed in at peripheral margin of the tumor so that the tumor tissue and normal parenchyma were ablated simultaneously (Fig. 1A). RF energy was applied for five minutes with the generator output titrated to maintain a designated tip temperature: a mean temperature of 90 ± 2℃. RF ablation was performed in random order by an investigator who was unaware of the treatment groups.
Euthanasia of animals was performed with pentobarbital after RF ablation immediately, and the livers were excised. Each tumor was sectioned at 2-3 mm intervals in a cross-sectional fashion, perpendicular to the line of the RF electrode tract. The representative slices were soaked in 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Fisher Scientific, Fairlawn, NJ, USA) for 30 minutes. With this method, viable tissue with intact mitochondrial enzyme activity stains red, while ablated tissue does not turn red. The absence of mitochondrial enzyme activity has been shown to accurately reveal irreversible cellular injury by percutaneous tumor ablation. The ablation areas were measured with use of software (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA) (Fig. 1B). Overall ablation area included tumor ablation area and non-tumorous parenchymal ablation area (Fig. 1C). The ratio of tumoral ablation area to overall ablation area was calculated to verify whether the effect of As2O3 on RF ablation area has selectivity for liver tumor. The ablation areas of the three groups were compared with Kruskal-Wallis and Mann-Whitney U test. The p value < 0.05 was considered significant. Microscopic examination was also performed in all specimens with Hematoxylin and Eosin staining. All the histologic evaluations were performed by a pathologist unaware of the experimental groups.
Gross examination showed the ablation lesion in each specimen as oval-shaped paled area of ablation necrosis including the VX2 tumor and adjacent normal liver parenchyma, the central tissue loss areas around the needle track, and peripheral thin dark-brown hemorrhagic rim (Fig. 2).
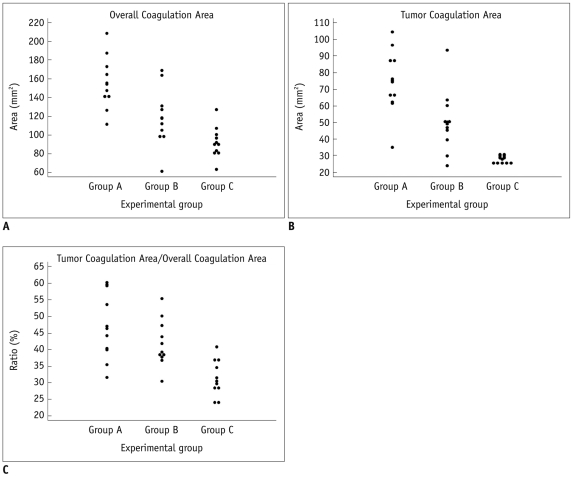
The ablation areas on TTC stained specimen were graphed in Figure 3A. The overall ablation areas were measured as 111.7-208.7 mm2 (mean, 156 ± 28.9 mm2; median, 151.0 mm2) in group A, 61.5-169.2 mm2 (mean, 119 ± 31.7 mm2; 115.2 mm2) in group B, 63.4-127.5 mm2 (mean, 92 ± 17.4 mm2; 90.4 mm2) in group C. The difference in overall ablation areas of the three groups were statistically significant (p < 0.001). Overall ablation area of group A was larger than those of group B (p = 0.019) and group C (p < 0.001). The difference between group B and group C was also statistically significant (p = 0.029) (Fig. 3A).
Tumoral ablation areas were measured as 35.2-104.4 mm2 (mean, 73 ± 19.7 mm2; 70.9 mm2) in group A, 24.1-93.6 mm2 (mean, 50 ± 19.4 mm2, 48.2 mm2) in group B, 25.8-30.8 mm2 (mean, 28 ± 2.2 mm2, 28.9 mm2) in group C. The tumoral ablation area of group A was significantly larger than those of group B (p = 0.019) and group C (p < 0.001). The difference between group B and C was not statistically significant (p = 0.247) (Fig. 3B).
The ratios of tumoral ablation area to overall ablation area were calculated as 32-60% (mean, 47 ± 10.5%; median, 45%) in group A, 31-55% (mean, 42 ± 7.3%; 39%) in group B, 24-41% (mean, 32 ± 5.6%; 30%) in group C. The difference among the ratios of the three groups were statistically significant (p = 0.002). The ratios of group A and B were significantly larger than that of group C (p = 0.001 and 0.003, respectively). However, the difference between group A and B was not statistically significant (p = 0.218) (Fig. 3C).
To overcome the heat sink effect, there have been various trials to extend the area of the ablation including the hepatic artery embolization, interstitial saline injection, and pharmacologic modulation (5-9). Pharmacologic approaches aim to reduce hepatic arterial flow to the tumor and to prevent heat dissipation, and subsequently augment the area of ablation necrosis induced by RF ablation. Therefore the ideal agent for this approach should be able to reduce blood flow of the target tumor selectively and preserve blood flow of non-tumoral hepatic parenchyma. In recent investigations, several drugs including halothane and As2O3 reduced tumoral blood flow selectively and expanded the RF ablation area in the VX2 liver and renal tumor models (9).
Although the action mechanism of As2O3 is not fully understood, previous studies showed that single or multiple doses of 8-10 mg/kg of As2O3 cause marked tumor vascular shutdowns and increased the anti-tumor effect of hyperthermic and radiation therapy in experimental solid tumors (15, 22, 23). Therefore, As2O3 is a promising pharmacologic modulator to overcome the limitation of hyperthermic ablation therapy (9, 17, 18). A recent study by Hines-Peralta et al. (17) found intravenous As2O3 administration induced larger ablation necrosis in a liver tumor model. This study is in accordance with their results demonstrating a larger ablation area in the intravenous As2O3 group compared with the control group (118.7 mm2 versus 63.4 mm2) (9, 14, 15, 17). Furthermore, we found that significant augmentation of the RF ablation area could be achieved by intraarterial As2O3 administration as well as by intravenous administration.
In our study, the intraarterial group showed a larger ablation area than those of the intravenous group (155.8 mm2 versus 118.7 mm2). This result might is not unexpected considering that trans-arterial treatment is generally accepted as a more effective approach than systemic treatments in liver tumors (24). In a biodistribution study employing intravenous administration of As2O3, the tissue concentration of monomethylarsinic acid, a major metabolite of As2O3, was highest in the lung and kidney, suggesting most of systemically administered As2O3 is metabolized in organs other than the liver (25). Thus, we think that hepatic arterial administration of As2O3 is a more favorable strategy than the intravenous approach to increase the local concentration and avoid the toxicity in treatment of liver tumors.
Arsenic trioxide has a very narrow therapeutic window. A dose of As2O3 more than 5 mg/kg shows signs of liver and kidney toxicity when administered intraperitoneally or intravenously. Conversely, a low dose of As2O3 less than 1 mg/kg shows an opposite effect including the promotion of tumor growth, up-regulated tumor angiogenesis and no effect on tumor cell apoptosis (26, 27). Thus, it is important to determine the dose of As2O3 and the timing of the RF ablation after As2O3 administration. Based on a previous study employing intravenous administration of As2O3 in rabbit model (17), we adopted 1 mg/kg of As2O3 and ablated the tumor one hour after As2O3 administration. We didn't confirm the optimal dose and temporal window in the intraarterial administration because this study did not include a dose escalation study component. However, despite an optimal dose and timing of intravenous As2O3 administration larger ablation was achieved in the intraarterial group. Therefore, we believe that a bigger difference between intravenous and intraarterial groups would be achieved if the optimal dose and timing for intraarterial administration was used.
During the RF ablation procedure, we intentionally located the RF electrode at the margin of the VX2 tumor to simultaneously induce ablation necrosis in both tumor and normal liver parenchyma (Fig. 1). This technique was made to determine whether As2O3 has a selective effect on tumor tissue. The ratio of the tumor ablation area to overall ablation area was used as an index of tumor selectivity. The ratios of the intraarterial and intravenous group were significantly higher than that of the control group. These results support the previous report which showed a greater decrease of tumoral blood flow than normal parenchymal flow after As2O3 administration (17). Therefore, As2O3 seems to have a selective anti-vascular effect on tumor tissue. This result suggests pharmacologic modulation using As2O3 as a favorable technique to augment RF ablation compared to other strategies such as hepatic artery embolization and interstitial saline injection. From a clinical aspect, combined treatment with As2O3 and other chemoembolic materials should be investigated for the radiofrequency ablation procedure because trans-arterial chemoembolization with radiofrequency ablation is already used in practice.
This study has important limitations. First, the optimal dose of As2O3 and temporal window for RF ablation was not evaluated. Further studies employing various doses and time intervals should be performed to find the optimal setting for intraarterial As2O3 administration. Second, we evaluated tumoral selectivity of As2O3 effect comparing tumoral and non-tumoral ablation areas. However, this method can be influenced by many factors such as exact position of the electrode and presence of adjacent large vessels. We think this limitation can be overcome by employing methods to measure tumoral blood flow directly such as laser Doppler flowmetry. Third, we did not perform tumor ablation and normal liver parenchymal ablation with an independent ablation method. Because the two ablations were impossible to separate for small rabbit liver, we intentionally targeted the tumor margin to perform the simultaneous ablation and to reduce the number of experimental animals. Fourth, we only have analyzed the ablation zone by a two dimensional pathologic method, but the three-dimensional analysis of the ablation zone would be necessary to evaluate the heat sink effect.
In conclusion, As2O3 administration selectively augments RF-induced ablation in a VX2 liver tumor. Hepatic arterial administration is a favorable approach to induce larger ablation than intravenous administration. Further studies are recommended to verify the optimal dose and temporal window for intraarterial administration of As2O3.
References
1. Lencioni R, Crocetti L. Image-guided thermal ablation of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008; 66:200–207. PMID: 18304832.

2. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89. PMID: 18008357.

3. Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound. 2001; 13:149–158. PMID: 11369526.
4. Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998; 9:101–111. PMID: 9468403.

5. Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000; 217:119–126. PMID: 11012432.

6. Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999; 20:47–53. PMID: 10407974.

7. McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996; 3:418–422. PMID: 8796695.

8. Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997; 202:205–210. PMID: 8988212.

9. Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, et al. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol. 2004; 15:269–274. PMID: 15028812.

10. Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001; 6(Suppl 2):3–10. PMID: 11331434.

11. Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001; 6(Suppl 2):22–28. PMID: 11331437.

12. Tamm I, Paternostro G, Zapata JM. Treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1999; 340:1043. author reply 1044-1045. PMID: 10189285.

13. Tan B, Huang JF, Wei Q, Zhang H, Ni RZ. Anti-hepatoma effect of arsenic trioxide on experimental liver cancer induced by 2-acetamidofluorene in rats. World J Gastroenterol. 2005; 11:5938–5943. PMID: 16273603.

14. Xu HY, Yang YL, Liu SM, Bi L, Chen SX. Effect of arsenic trioxide on human hepatocarcinoma in nude mice. World J Gastroenterol. 2004; 10:3677–3679. PMID: 15534931.

15. Griffin RJ, Monzen H, Williams BW, Park H, Lee SH, Song CW. Arsenic trioxide induces selective tumour vascular damage via oxidative stress and increases thermosensitivity of tumours. Int J Hyperthermia. 2003; 19:575–589. PMID: 14756449.

16. Monzen H, Griffin RJ, Williams BW, Amano M, Ando S, Hasegawa T. Study of arsenic trioxide-induced vascular shutdown and enhancement with radiation in solid tumor. Radiat Med. 2004; 22:205–211. PMID: 15468939.
17. Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology. 2006; 240:82–89. PMID: 16720872.

18. Hakime A, Hines-Peralta A, Peddi H, Atkins MB, Sukhatme VP, Signoretti S, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology. 2007; 244:464–470. PMID: 17641366.

19. Sze DY, Freeman SM, Slonim SM, Samuels SL, Andrews JC, Hicks M, et al. Dr. Gary J. Becker Young Investigator Award: intraarterial adenovirus for metastatic gastrointestinal cancer: activity, radiographic response, and survival. J Vasc Interv Radiol. 2003; 14:279–290. PMID: 12631632.

20. Hohn DC, Stagg RJ, Friedman MA, Hannigan JF Jr, Rayner A, Ignoffo RJ, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989; 7:1646–1654. PMID: 2530317.

21. Institute of Laboratory Animal Resources Committee on Revision of the Guide for Laboratory Animals Facilities and Care of the Institute of Laboratory Animal Resources National Research Council. Guide for laboratory animal facilities and care. 1968. 3rd revised ed. Washington, DC: U.S.: Public Health Service Publication;No. 1024, 19681968.
22. Lew YS, Brown SL, Griffin RJ, Song CW, Kim JH. Arsenic trioxide causes selective necrosis in solid murine tumors by vascular shutdown. Cancer Res. 1999; 59:6033–6037. PMID: 10626785.
23. Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP Jr, et al. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000; 96:1525–1530. PMID: 10942401.

25. Lin CJ, Wu MH, Hsueh YM, Sun SS, Cheng AL. Tissue distribution of arsenic species in rabbits after single and multiple parenteral administration of arsenic trioxide: tissue accumulation and the reversibility after washout are tissue-selective. Cancer Chemother Pharmacol. 2005; 55:170–178. PMID: 15322825.

26. Liu B, Pan S, Dong X, Qiao H, Jiang H, Krissansen GW, et al. Opposing effects of arsenic trioxide on hepatocellular carcinomas in mice. Cancer Sci. 2006; 97:675–681. PMID: 16827809.

27. Wang SS, Zhang T, Wang XL, Hong L, Qi QH. Effect of arsenic trioxide on rat hepatocellular carcinoma and its renal cytotoxity. World J Gastroenterol. 2003; 9:930–935. PMID: 12717832.
Fig. 1
Experimental concept of intentional marginal ablaion.
A. Radiofrequency electrode (small black dot) was placed at peripheral margin of tumor to simultaneously induce tumor and hepatic parenchymal ablation. B. Overall ablation area. Illustration showing overall ablation area (white circle) includes tumor ablation area and hepatic parenchymal area. C. Selectivity of As2O3 effect. Illustration showing coagulated tumor area (A) and coagulated hepatic parenchymal area (B). Each area was measured to verify whether selective antivascular property of As2O3 on liver tumor has influence on radiofrequency ablation. Radios of coagulated tumor area versus coagulated hepatic parenchymal area were calculated.
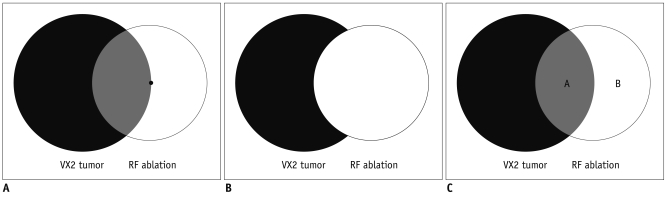
Fig. 2
Triphenyltetrazolium chloride stained specimens. With triphenyltetrazolium chloride staining, viable tissue with intact mitochondrial enzyme activity stains red, while ablated tissue remains white. Arrow indicating VX2 carcinoma and arrowheads point to ablated area. Ablated area of specimen from group A is larger than that from group B and C.
A. Specimen from group A administered through hepatic artery. B. Specimen from group B administered through auricular vein. C. Specimen from group C, control group, administered phosphate-buffered saline through auricular vein.
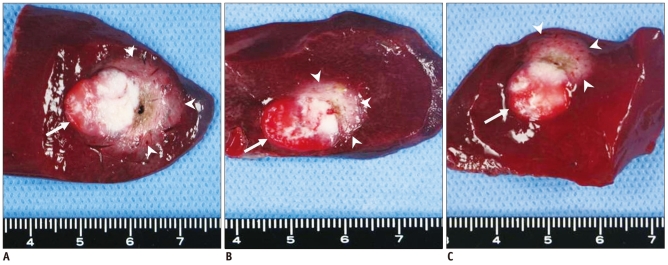
Fig. 3
Result of tumor ablation in each experimental group.
A. Graph demonstrating overall ablation area in each group confirmed by triphenyltetrazolium chloride staining. Ablation was performed one hour after administration of arsenic trioxide in each group. Overall ablation area of group A was larger than that of group B (p < 0.01) and C (p < 0.001). Difference between group B and C was also statistically significant (p < 0.04). B. Graph demonstrating coagulated tumor area in each group. Ablation tumor area in group A was significantly larger than that of group B (p = 0.02) and C (p < 0.01). Difference between group B and C was not statistically significant. C. Graph demonstrating ratio tumor ablation of overall ablation area in each group, confirmed by triphenyltetrazolium chloride staining.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download