Abstract
Objective
Upper gastrointestinal (GI) bleeding is a serious complication that sometimes occurs after percutaneous radiologic gastrostomy (PRG). We evaluated the incidence of bleeding complications after a PRG and its management including transcatheter arterial embolization (TAE).
Materials and Methods
We retrospectively reviewed 574 patients who underwent PRG in our institution between 2000 and 2010. Eight patients (1.4%) had symptoms or signs of upper GI bleeding after PRG.
Results
The initial presentation was hematemesis (n = 3), melena (n = 2), hematochezia (n = 2) and bloody drainage through the gastrostomy tube (n = 1). The time interval between PRG placement and detection of bleeding ranged from immediately after to 3 days later (mean: 28 hours). The mean decrease in hemoglobin concentration was 3.69 g/dL (range, 0.9 to 6.8 g/dL). In three patients, bleeding was controlled by transfusion (n = 2) or compression of the gastrostomy site (n = 1). The remaining five patients underwent an angiography because bleeding could not be controlled by transfusion only. In one patient, the bleeding focus was not evident on angiography or endoscopy, and wedge resection including the tube insertion site was performed for hemostasis. The other four patients underwent prophylactic (n = 1) or therapeutic (n = 3) TAEs. In three patients, successful hemostasis was achieved by TAE, whereas the remaining one patient underwent exploration due to persistent bleeding despite TAE.
Gastrostomy is a well-established procedure for long-term nutritional support or gastric decompression in patients incapable of oral intake due to various disorders (1, 2). Gastrostomy may be performed surgically or percutaneously, under either endoscopic or fluoroscopic guidance (1). Percutaneous radiologic or endoscopic gastrostomy (PRG or PEG) is less invasive and has lower complication rates than surgical methods. The incidence of major complications after PRG or PEG has been found to range from 0 to 6% (3-5).
Upper gastrointestinal (GI) bleeding complicating gastrostomy is rare, but may be catastrophic when it occurs (6-11). Most patients who experience gastric bleeding have undergone surgical gastrostomy or PEG (7-11), with few reports focusing on bleeding complications after PRG. Furthermore, even less is known about the use of transcatheter arterial embolization (TAE) to treat gastrostomy-related upper GI bleeding (8, 10). We therefore assessed the incidence of bleeding complications after PRG and their management, including TAE.
From September 2000 to September 2010, 574 patients underwent PRG at our institution. The gastrostomy was most commonly indicated in patients with swallowing difficulties due to neurological disorders or head and neck malignancy. Further indications were in those patients who need additional nutritional support due to chronic illness or intestinal malabsorption and less commonly in those patients who require gastric decompression. Contraindications included interposition of the colon and the liver between the stomach and the anterior abdominal wall, previous gastric surgery, presence of ascites, and severe uncontrollable coagulopathy. Screening for bleeding tendency was performed before PRG procedure and acceptable coagulation parameters at our institution were a prothrombin time over 60 percent and a platelet count exceeding 50000 i.u. Although ceasing any anticoagulant medications at the time of procedure was strongly recommended, correction of coagulopathy was enough in those patients who should continue their medications.
We searched the records for patients who had symptoms or signs of upper GI bleeding after PRG, including hematemesis, melena, hematochezia or bloody drainage from the gastrostomy tube (11). Of these 574 patients, 8 (1.4%) had episodes of upper GI bleeding. They included 6 men and 2 women, ranging in age from 32-87 years (mean age, 69 years). PRG was performed for head and neck malignancy in 3 patients, cerebral infarction in 2, and neuromuscular disorder, vascular dementia with dysphagia, nutritional support after surgical repair of aortic dissection in 1 patient each.
The stomach was inflated with about 300-400 mL of air, using a previously inserted nasogastric tube or a 5-F Cobra catheter (Cook, Bloomington, IN, USA) previously inserted under fluoroscopic guidance. A puncture site equidistant from the greater and lesser curvatures of the stomach was selected at the distal gastric body, with one anchor (Cope gastrointestinal suture anchor sets; Cook) used for gastropexy. Interposition of the left hepatic lobe and the colon were carefully avoided using ultrasound and an air enema. After local anesthesia with 1% lidocaine, a 17-gauge needle with a preloaded anchor or a 21-gauge Chiba needle was inserted into the stomach through the skin incision site, and the intragastric position of the needle was confirmed by contrast injection. The Chiba-needle was then exchanged for a 6-Fr Neff catheter (Cook), and an anchor was deployed into the stomach lumen through the Neff catheter using a 0.035-inch superstiff guide wire for the gastropexy. When the stomach and abdominal wall were approximated, the tract was dilated over the guide-wire to enable placement of a 14-Fr diameter loop catheter (Cook), followed by injection of contrast material through the catheter to confirm its location.
A 5-Fr end-hole catheter was introduced over a 0.035-inch guide-wire (Terumo; Radifocus, Tokyo, Japan) via the right femoral artery. Superior mesenteric, common hepatic and left gastric angiograms were performed in all patients. If there was evidence of active bleeding on the angiogram, defined as extravasation of contrast medium or the presence of a pseudoaneurysm (12), TAE was performed using a microcatheter by one of two interventional radiologists with 12-15 years of experience. Embolic materials included gelfoam slurry, microcoils, or N-butyl cyanoacrylate (NBCA). After TAE, a completion angiogram was obtained to confirm the cessation of bleeding or occlusion of the target arteries.
Demographic data, initial presentation, time interval between PRG placement and detection of bleeding, possible causes of bleeding, administration of anticoagulants, decrease in hemoglobin (Hb) level, management of bleeding, and clinical outcomes were recorded for all eight patients by review of their electronic medical records, radiologic methods (angiography, CT, tubography), and endoscopic findings.
Of the eight patients, three initially presented with hematemesis, followed by two with melena, two with hematochezia and one with bloody drainage through the gastrostomy tube. The time interval between PRG placement and detection of bleeding ranged from immediately after to 3 days later (mean, 27.8 h), with bleeding within 24 hours observed in five patients. Seven patients experienced bleeding after their initial PRG, with one (No. 6) experiencing PRG after PEG failure. The eighth patient (No. 4) experienced bleeding after reinsertion of the PRG tube following self-removal of a previously inserted tube. Of 574 patients, 56 (9.8%) took anticoagulant medications at the time of the PRG procedure, and 2 of 56 patients (3.6%, No. 5, 8) on anticoagulants had bleeding complications. There was no significant difference in the incidence of bleeding complications between the two groups with and without anticoagulants (p = 0.179, Chi-square test using SPSS). Two patients (No. 5, 8) with cerebral infarction and one (No. 2) with a covered stent for a common carotid artery pseudoaneurysm (due to radiation therapy for nasopharyngeal cancer) had received anticoagulant therapy, and the patient with the covered stent stopped this medication one week before PRG. The mean decrease in Hb concentration was 3.69 g/dL (range, 0.9 to 6.8 g/dL).
Of the eight patients with bleeding complications, seven were given blood transfusions as an initial therapy (Table 1), with two recovering after transfusion. The one patient (No. 8) who did not require a blood transfusion had a small amount of bloody drainage immediately after the PRG, which stopped after compression of the gastrostomy site. He had been taking aspirin and warfarin for the treatment of a cerebral infarction at the time of the procedure.
Five patients required endoscopy as well as angiography as the next step in conservative management. Of the five patients who underwent angiography, two (No. 1, 5) showed no bleeding focus. One (No. 1) with massive GI bleeding and hypotension underwent prophylactic TAE of both gastric and right gastroepiploic arteries with gelfoam slurry, resulting in successful hemostasis. A subsequent endoscopy demonstrated hemorrhagic gastritis, which was regarded as resulting from irritation by the tube. No further bleeding was seen on endoscopy. The other patient (No. 5) showed substantial volumes of fresh blood and blood clots without demonstration of a bleeding focus on an endoscopy performed 12 hours after undergoing an angiography. Two days later, this patient underwent wedge resection including the tube insertion site for bleeding control. The tube insertion site was clear and a pathologic examination showed angiodysplasia in the resected stomach.
Of the five patients who underwent angiography, three (No. 2-4) showed contrast extravasation. One (No. 2) who underwent covered stent insertion for a carotid artery pseudoaneurysm two weeks before PRG had an active bleeding focus in a branch of the right gastroepiploic artery, as well as multiple mycotic pseudoaneurysms in branches of the superior mesenteric artery, probably due to septic emboli (Fig. 1). TAE with NBCA was performed after selection of the bleeding focus at the common hepatic artery level. Another patient (No. 3) showed active bleeding from a branch of the short gastric artery. Because superselection of the branch was impossible, TAE with gelfoam slurry and microcoils was performed at the origin of the branch from the short gastric artery. A subsequent endoscopy showed no active bleeding after embolization. The third patient (No. 4), who had undergone surgical repair from an aortic dissection, showed an active bleeding focus at the tube insertion site on endoscopy. After tube removal, epinephrine and fibrin glue were injected with hemoclipping endoscopically, but bleeding was not controlled. A subsequent angiography showed an active bleeding focus at the greater curvature of the stomach (Fig. 2). It was impossible to advance a microcatheter directly into the bleeding site due to a very tortuous course. TAE with gelfoam slurry and microcoils was performed at the proximal part of the right gastroepiploic artery. While post-TAE angiogram showed no further bleeding, bloody L-tube irrigation lasted and an emergency exploration was performed. The surgeons observed a tear of the right gastroepiploic artery, which was subsequently ligated. Although bleeding control was achieved, this patient died five weeks later due to surgical wound problems and sepsis.
We found that the incidence of complications from upper GI bleeding after PRG was 1.4% (8/574) and that the incidence of major bleeding complications requiring transfusion was 1.2% (7/574). In comparison, other studies have reported that the incidence of major bleeding complications after percutaneous radiologic gastrostomy or gastroenterostomy was 0.7-3.0% (6, 7, 13), including 1 of 130 patients (0.7%) with major gastric hemorrhage complicating the percutaneous transgastric jejunostomy (7), 5 of 158 (3%) with major GI bleeding after percutaneous radiologic gastrostomy or gastroenterostomy (6), and 4 of 216 (1.9%) with major bleeding after PRG with the one-anchor technique of gastropexy (13). Similar incidences of bleeding complications have been reported after PEG (11, 14-18), including in 2 of 314 (0.6%) (15) and 2 of 232 (0.9%) patients (14) with major bleeding after installing PEG, and in 17 of 338 (4.1%) with episodes of upper GI bleeding after the PEG installation, including 14 with minor bleeding who did not require transfusions (11). Following surgical gastrostomy, major gastric hemorrhage requiring transfusion was observed in 4 of 424 (0.9%) and in 2 of 147 (1.4%) patients (19, 20), indicating that the rates of major bleeding complications are similar after radiologic, endoscopic, and surgical gastrostomy.
Although the incidence of bleeding complications following gastrostomy is not high, managing such complications is important because of their potentially serious consequences, which include death (6, 10, 14). Among the available treatment options are conservative management, endoscopic hemostasis, TAE, and surgery. Conservative management of gastrostomy-related bleeding with blood transfusion has been reported (6, 9, 18, 21). For example, three of five patients with bleeding recovered uneventfully after transfusion (6), and one HIV-seropositive (9) with upper GI bleeding following PEG was successfully managed by transfusion. Another patient with PEG-associated upper GI bleeding recovered after a transfusion, who presented with focal erythema and a clot at the gastrostomy site without active bleeding (21). We found that upper GI bleeding in three of our eight patients was controlled by transfusion or local compression.
Endoscopic interventions such as sclerotherapy or hemoclip placement have been shown effective for managing gastrostomy-related upper GI bleeding (11). Endoscopy is superior in localizing the bleeding site, characterizing the cause of bleeding, and showing the relationship between the bleeding site and the gastrostomy tube. Endoscopy, however, may be limited by several factors, including the presence of comorbid illnesses, active bleeding, bleeding vessels larger than 2 mm, and endoscopic blind spots, all of which may increase hemostatic failure for upper GI bleeding (22, 23). In our study, one patient (No. 4) showed persistent bleeding even after fibrin glue injection and endoscopic clipping because of profuse bleeding from a surgically confirmed gastric artery tear. Patients with a failed endoscopic intervention should be considered for TAE or surgical treatment.
TAE is widely accepted for the management of upper GI bleeding, especially in patients who fail conservative or endoscopic therapy, and is generally preferred over surgery in high-risk patients. There have been a few reports about TAE treatment for bleeding complications after PEG (8, 10). For example, occlusion of both gastroepiploic arteries by selective TAE successfully stopped bleeding in a patient with unstable hemodynamic status after PEG (8), and TAE of the left gastric artery was successful in stopping massive bleeding after PEG, although that patient died of multi-organ failure despite successful hemostasis (10). In contrast, less is known about the use of TAE for major bleeding after PRG (13). We found that TAE successfully controlled bleeding in three of four patients. In the remaining patient (No. 4), who showed persistent bleeding after TAE, TAE was possible only in the proximal part of the bleeding focus because the microcatheter could not be further advanced. The persistent bleeding from the distal part of the bleeding focus was finally managed with surgery. If both the proximal and distal parts of the bleeding focus cannot be securely embolized, there is the potential for rebleeding. Generally, surgery is a salvage method for patients in whom bleeding cannot be controlled by endoscopy and/or angiography.
Blind or prophylactic TAE, defined as TAE without angiographic evidence of bleeding, may be beneficial (24-27). As massive bleeding is sometimes intermittent (28) and TAE in the upper GI tract is generally considered safe, blind TAE for upper GI bleeding is recommended when guided endoscopically (25-27). Indeed, one of our patients (No. 1) with no angiographically determined bleeding focus was successfully treated by prophylactic TAE of both gastric and right gastroepiploic arteries with gelfoams.
The causes of bleeding after PEG include the direct puncture of blood vessels, traumatic erosion of the mucosa, and ulceration induced by an internal bolster (29). Among the 17 patients who developed upper GI bleeding after PEG, reflux esophagitis was the most common cause, followed by gastric ulcer and gastric erosions (11). That study found that the time from PEG to bleeding was usually more than 3 days (mean, 308 days). In contrast, we found that the time interval between PRG and detection of bleeding was always less than 3 days (mean, 33.4 h). A longer time interval suggests that bleeding in these patients is due to irritation or ulceration by an internal bolster. In both of our patients (No. 1, 5) with endoscopically or pathologically determined underlying hemorrhagic gastritis or angiodysplasia, mucosal irritation or injury induced by tubes or gastrostomy devices may have resulted in bleeding after PRG. In one patient (No. 8) with a small amount of bloody drainage, which ceased after compression of the gastrostomy site, anticoagulants would have provoked oozing blood from the gastrostomy site. Direct puncture of right gastroepiploic artery would be the cause of bleeding in one patient (No. 4) as a tear of the vessel was confirmed at the operating field. For the remaining four patients including two patients (No. 2, 3) whose angiography showed extravasation, we suggest that a traumatic injury of the vessels could be the cause of bleeding.
This study has limitations. First, it is a retrospective review without randomization. Second, the number of study patients with major bleeding was small; however, they were collected from 574 patients who underwent a PRG over a 10-year period.
In conclusion, we found that the incidence of upper GI bleeding after PRG was 1.4%, which is comparable to previous reports. TAE seems to be safe and effective in achieving hemostasis for bleeding after PRG, as the next step after conservative management.
Figures and Tables
Fig. 1
Thirty two-year-old male patient with nasopharyngeal cancer (patient No. 2).
A. Superior mesenteric angiogram shows extravasation from branch of right gastroepiploic artery (arrows) adjacent to tube. Pseudoaneurysm (arrowhead) in splenic artery caused by septic emboli is also observed. Due to retrograde flow with celiac stenosis, gastroepiploic artery and splenic artery are visualized on superior mesenteric angiogram. B. Successful N-butyl cyanoacrylate embolization (arrows) was done by superselection of bleeding focus at common hepatic artery level.
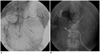
Fig. 2
Eighty seven-year-old female who previously underwent surgical repair of aortic dissection (patient No. 4).
A. Right gastroepiploic angiogram shows extravasation (arrows) at greater curvature of stomach near hemoclips (arrowhead). B. Embolization with gelfoam slurry and microcoils (arrow) was performed at proximal part of bleeding focus due to marked tortuosity of right gastroepiploic artery. Splenic angiogram shows no collateral flow from left gastroepiploic artery (not shown). Although post-embolization angiogram shows no further bleeding, patient underwent laparotomy due to uncontrolled bleeding.

References
1. Laasch HU, Wilbraham L, Bullen K, Marriott A, Lawrance JA, Johnson RJ, et al. Gastrostomy insertion: comparing the options--PEG, RIG or PIG? Clin Radiol. 2003. 58:398–405.
2. Dewald CL, Hiette PO, Sewall LE, Fredenberg PG, Palestrant AM. Percutaneous gastrostomy and gastrojejunostomy with gastropexy: experience in 701 procedures. Radiology. 1999. 211:651–656.
3. Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005. 56:84–90.
4. Möller P, Lindberg CG, Zilling T. Gastrostomy by various techniques: evaluation of indications, outcome, and complications. Scand J Gastroenterol. 1999. 34:1050–1054.
5. Wollman B, D'Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995. 197:699–704.
6. Hicks ME, Surratt RS, Picus D, Marx MV, Lang EV. Fluoroscopically guided percutaneous gastrostomy and gastroenterostomy: analysis of 158 consecutive cases. AJR Am J Roentgenol. 1990. 154:725–728.
7. Rose DB, Wolman SL, Ho CS. Gastric hemorrhage complicating percutaneous transgastric jejunostomy. Radiology. 1986. 161:835–836.
8. Lewis MB, Lewis JH, Marshall H, Lossef SV. Massive hemorrhage complicating percutaneous endoscopic gastrostomy: treatment by means of transcatheter embolization of the right and left gastroepiploic arteries. J Vasc Interv Radiol. 1999. 10:319–323.
9. Cappell MS, Godil A. A multicenter case-controlled study of percutaneous endoscopic gastrostomy in HIV-seropositive patients. Am J Gastroenterol. 1993. 88:2059–2066.
10. Youn BJ, Hur J, Lee KH, Won JY. Transarterial embolization of massive gastric ulcer bleeding in gastrostomy patients caused by a balloon teplacement tube: a Case Report. J Korean Radiol Soc. 2007. 56:137–139.
11. Nishiwaki S, Araki H, Takada J, Watanabe N, Asano T, Iwashita M, et al. Clinical investigation of upper gastrointestinal hemorrhage after percutaneous endoscopic gastrostomy. Dig Endosc. 2010. 22:180–185.
12. Shin JH, Song HY, Kim TH, Kim KR, Choi KE, Kim JH. Percutaneous radiologic gastrostomy: a modified Chiba-needle puncture technique with single gastropexy. Abdom Imaging. 2010. 35:189–194.
13. Kim JW, Song HY, Kim KR, Shin JH, Choi EK. The one-anchor technique of gastropexy for percutaneous radiologic gastrostomy: results of 248 consecutive procedures. J Vasc Interv Radiol. 2008. 19:1048–1053.
14. Amann W, Mischinger HJ, Berger A, Rosanelli G, Schweiger W, Werkgartner G, et al. Percutaneous endoscopic gastrostomy (PEG). 8 years of clinical experience in 232 patients. Surg Endosc. 1997. 11:741–744.
15. Larson DE, Burton DD, Schroeder KW, DiMagno EP. Percutaneous endoscopic gastrostomy. Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology. 1987. 93:48–52.
16. Rabeneck L, Wray NP, Petersen NJ. Long-term outcomes of patients receiving percutaneous endoscopic gastrostomy tubes. J Gen Intern Med. 1996. 11:287–293.
17. Mamel JJ. Percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1989. 84:703–710.
18. Rimon E. The safety and feasibility of percutaneous endoscopic gastrostomy placement by a single physician. Endoscopy. 2001. 33:241–244.
19. Shellito PC, Malt RA. Tube gastrostomy. Techniques and complications. Ann Surg. 1985. 201:180–185.
20. Wasiljew BK, Ujiki GT, Beal JM. Feeding gastrostomy: complications and mortality. Am J Surg. 1982. 143:194–195.
21. Cappell MS, Iacovone FM Jr. The safety and efficacy of percutaneous endoscopic gastrostomy after recent myocardial infarction: a study of 28 patients and 40 controls at four university teaching hospitals. Am J Gastroenterol. 1996. 91:1599–1603.
22. Cheung FK, Lau JY. Management of massive peptic ulcer bleeding. Gastroenterol Clin North Am. 2009. 38:231–243.
23. Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008. 103:2625–2632. quiz 2633.
24. Lee HJ, Shin JH, Yoon HK, Ko GY, Gwon DI, Song HY, et al. Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol. 2009. 19:960–965.
25. Burke SJ, Golzarian J, Weldon D, Sun S. Nonvariceal upper gastrointestinal bleeding. Eur Radiol. 2007. 17:1714–1726.
26. Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001. 12:195–200.
27. Morris DC, Nichols DM, Connell DG, Burhenne HJ. Embolization of the left gastric artery in the absence of angiographic extravasation. Cardiovasc Intervent Radiol. 1986. 9:195–198.
28. Sos TA, Lee JG, Wixson D, Sniderman KW. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: arteriographic demonstration. AJR Am J Roentgenol. 1978. 131:1015–1017.
29. McClave SA, Chang WK. Complications of enteral access. Gastrointest Endosc. 2003. 58:739–751.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download