Abstract
Objective
We investigated low dose digital tomosynthesis (DT) for the evaluation of the paranasal sinus (PNS), and compared its diagnostic accuracy with a PNS radiography series (XR).
Materials and Methods
We enrolled 43 patients for whom XR, PNS DT, and OMU CT were performed. We measured effective doses (EDs) of XR, DT, and OMU CT using Monte Carlo simulation software. Two radiologists performed independent observation of both XR and DT. For seven PNSs, they scored anatomic conspicuity of sinuses and confidence on the presence of sinusitis using nine point scales. OMU CT was observed by the third radiologist and the findings were regarded as reference standard. We compared scores for conspicuity and sinusitis confidence between XR and DT.
Results
Mean EDs were 29 ± 6 µSv, 48 ± 10 µSv, and 980 ± 250 µSv, respectively, for XR, DT, and CT. Mean scores for conspicuity were 6.3 and 7.4, respectively, for XR and DT. Sensitivity per patient basis for sinusitis detection were 52% and 96%, respectively, for XR and DT in observer 1 (p = 0.001) and 80% and 92% for observer 2 (p = 0.25). Specificities for sinusitis exclusion were 100% for both XR and DT for observer 1 and 89% and 100% for observer 2 (p = 0.50). Accuracies for sinusitis diagnosis were 72% and 98%, respectively, for XR and DT for observer 1 (p = 0.001) and 84% and 95% for observer 2 (p = 0.125).
Digital tomosynthesis (DT) is a form of limited-angle computed tomography that allows reconstruction of multiple section images from a set of projection data acquired over a limited range of X-ray tube angles (1). The term 'tomosynthesis' was defined by Grant in 1972 (2) by combining the two Greek words 'tomos'-a section, a slice, or a cutting-and 'synthesis'-a process, resulting in formation of something new. While the theory of tomosynthesis has been previously described, it has only recently been introduced as commercial equipment for medical imaging in the form of chest tomosynthesis (3). This new modality offers the potential for improved diagnostic performance over conventional radiography by substantially reducing the visual clutter of the overlying anatomy (1, 4-6). Although it does not have the depth resolution of CT, DT? provides high-resolution images in the coronal or sagittal plane with a substantial reduction in the radiation dose compared with CT (5-7).
Failure to detect sinusitis can lead to serious complications, such as cellulitis and osteomyelitis (8-10). Standard plain radiography, such as Caldwell or Water's views, used to be the primary imaging methods. These imaging methods do not have an acceptable diagnostic accuracy in cases of sinusitis. Therefore, a CT scan of the PNS is currently the imaging method of choice for sinusitis (8, 9, 11-15). A standard CT scan (SCT) of the PNS consists of coronal and axial sections. Because of these multiple slices, paranasal sinus (PNS) CT has a significant patient X-ray absorption dose (8, 13-15).
Although the radiation dose of DT is relatively higher than that of plain radiography (XR), DT is the most advanced digital radiography technology, which can be applied easily as an alternative to XR in many regions, including PNS evaluation.
Therefore, we investigated low dose (LD) PNS DT and compared its diagnostic accuracy for sinusitis? with that of PNS XR.
Our institutional review board approved this study, and all patients provided written informed consent. We enrolled 43 patients, for whom PNS DT, a PNS XR series, and OMU CT had been performed within one week and for whom images were available. From February of 2009 to March of 2010, we enrolled 43 consecutive patients aged 20-75 years (mean ± SD = 55 ± 18 years). Of the 43, 21 were males 55 ± 18 years) and were 22 females (56 ± 18 years).
An XR PNS series was performed using a commercial cesium iodide-amorphous silicon flat-panel detector digital radiography system (Definium 8000; GE Healthcare, Chalfont St Giles, England). Under automatic exposure control (AEC) with a detector speed of 400, two shot XR PNS series (Caldwell view and Water's view) were performed with 80kVp. We measured the entrance surface dose (ESD) using a glass dosimeter (Dose Ace, Asahi Technoglass, Japan). We calculated organ dose (OD) and effective dose (ED) using Monte Carlo simulation software (PCXMC v2.0, STUK, Helsinki, Finland).
Digital tomosynthesis examinations were conducted using a commercially available unit (Volume RAD; GE Healthcare) with cesium iodide-an amorphous silicon flat-panel detector system. We altered the DT parameters and established a lower radiation dose condition for PNS imaging. Under automatic exposure control conditions, DT was performed with 100 kVp, an 0.3 mm additional Copper filter, and a 1:5 dose ratio of the scout image.
In the same manner as for the XR PNS series, we calculated entrance surface dose (ESD), organ dose (OD), and effective dose (ED) by means of Monte Carlo simulation software (16).
In all patients, helical CT scans were obtained through the face, from the forehead to the maxilla, using 64-section equipment (LightSpeed VCT; GE Healthcare) with noncontrast studies. The scanning parameters were as follows: individual detector width, 0.625 mm; gantry rotation time, 600 msec; tube voltage, 120 kVp; tube current, 250 mA; and pitch, 0.97. Axial images were reconstructed using the following parameters: 1.25 mm section thickness, high-spatial-frequency reconstruction algorithm (bone preset), and an 18 cm field of view. Coronal images were reformatted from volume axial images with 2 mm intervals. Dose-length product (DLP) was recorded. The ED for OMU CT was calculated using a DLP to ED conversion factor of 2.2 µSv/(mGy-cm) (17).
Two radiologists did independent observations of both XR and DT and analyzed the image data separately. The two radiologists had different experiences; observer 1 had three years of CT experience and six months of DT experience, and observer 2 had sixteen years of CT experience and two years of DT experience.
Using nine point scales, they scored anatomic conspicuity and confidence of sinusitis for each sinus in each patient. Diagnostic criteria for both XR and DT for sinusitis were as follows: 1) Diffuse and/or polypoid mucoperiosteal thickening, 2) Total opacity of sinuses, 3) Air-fluid level, 4) Erosion and sclerosis of sinus walls.
All images were assessed using a picture archiving and communication system (Centricity RA 1000; GE Healthcare) and control of image conditions, such as window level/width or magnification, were fully permitted.
OMU CT served as the reference-standard method for the analysis. After completion of the detection study by the two observers, records from XR and DT images were matched and compared with those from OMU CT scan readings (both coronal and axial CT images). For comparison, two other board certified radiologists, with 20 and 12 years' experience with CT, reviewed the OMU CT and made conclusions by consensus.
We compared anatomic conspicuity between XR and DT using the score itself by Wilcoxon signed rank test because there is no reference standard for conspicuity. We analyzed the diagnostic performances of XR and DT, including their sensitivity, specificity, and accuracy for detection of sinusitis, by dividing the scores into two groups: positive (6-9) and negative (1-5). We compared diagnostic performance between XR and DT by the McNemar test. A commercially available software program was used for processing and analysis of data (PASW, version 17.0; SPSS, Chicago, IL, USA). P < 0.05 was considered to indicate statistical significance.
Receiver operator characteristic (ROC) analysis with calculation of the area under the ROC curve was performed using the ROCKIT program (Metz C, University of Chicago, Chicago, IL, USA).
EDs measured using a standard anthropomorphic phantom (female ART phantom; Radiology Support Devices, Long Beach, CA, USA) were 21 µSv, 26 µSv, and 910 µSv, respectively, for XR, DT, and CT. ODs for the brain were 0.39 mGy, 0.47 mGy, and 23 mGy, respectively, for XR, DT, and CT. Mean EDs of clinical cases were 29 ± 6 µSv, 48 ± 10 µSv, and 980 ± 250 µSv, respectively for XR, DT, and CT.
For observer 1, the scores for anatomic conspicuity for XR and DT for detection of PNS were 6.7 and 7.1 for the maxillary sinus (p = 0.108, Wilcoxon signed rank test), 6.1 and 6.3 for the ethmoid sinus (p < 0.001), 6.3 and 6.7 for the frontal sinus (p = 0.057), and 4.4 and 6.9 for the sphenoid sinus (p < 0.001), respectively. For observer 2, the corresponding scores were 7.0 and 8.5 for the maxillary sinus (p < 0.001), 6.7 and 8.1 for the ethmoid sinus (p < 0.001), 7.1 and 7.5 for the frontal sinus (p = 0.057), and 5.8 and 8.2 for the sphenoid sinus (p < 0.001).
Overall, for observer 1, mean scores for anatomic conspicuity for XR and DT for detection of PNS were 5.9 and 6.8, respectively. For observer 2, the corresponding mean scores were 6.7 and 8.1, respectively (Fig. 1).
Table 1 summarizes the diagnostic accuracy of XR and DT for detection of sinusitis in a person-based description. DT was? significantly superior than XR in the evaluation of person based sensitivities (52% for XR and 96% for DT; p = 0.001) and accuracy (72% for XR and 98% for DT; p = 0.001) for observer 1 (Fig. 2). However, there was no significant difference between XR and DR for observer 2.
Table 2 shows the diagnostic performance of XR and DT in the detection of sinusitis in seven sinuses. The overall sensitivities for XR and DT in a lesion-based description were 40% and 75% (p < 0.001) for observer 1 and 60% and 82% (p = 0.001) for observer 2. The overall specificities for XR and DT were 95% and 91% (p = 0.108) for observer 1 and 77% and 96% (p < 0.001) for observer 2. Moreover, overall accuracies of XR and DT were 79% and 86% (p < 0.001) for observer 1 and 72% and 92% (p = 0.031) for observer 2.
The areas under curves (Az) of the receiver operator characteristic (ROC) curve, which correspond to the accuracies for detection of paranasal sinusitis, were 0.700 and 0.891, respectively, for XR and DT for observer 1, and 0.813 and 0.924 for observer 2 (Fig. 3) (Table 3). Thus, these findings show that the accuracy of DT for detection of sinusitis is significantly higher (p < 0.05) than that of a PNS XR series.
Paranasal sinus radiography series are the first choice for diagnosis and follow-up for patients with paranasal sinusitis; however, low sensitivity and low specificity are major limitations (8, 9, 11-15). Problems related to limited sensitivity and specificity of radiography are alleviated with use of CT. However, higher doses of radiation and higher costs become problematic (13-15). A recently developed technique, DT, is an interesting alternative modality. The number of scientific papers on DT has increased over the years (1, 3, 5, 7, 18-25), which is an indicator of increasing interest. DT is associated with a low radiation dose compared with OMU CT, and with improved detection compared with PNS radiography (1, 6, 18, 26-32). From our results using a standard anthropomorphic phantom, the effective dose for PNS DT is approximately 0.06 mSv by manufacturer's default and 0.026 mSv by our low dose modification, which is approximately 3 times higher than that used for radiographic examination (0.01 mSv); however, it is approximately 30 times lower than that used for OMU CT examination (0.91 mSv) (6, 24). Usual PNS radiographic examination includes two or three exposures (Water's view, Caldwell view, and/or skull lateral view); thus, total exposure of DT will not exceed that of the PNS radiography series. Furthermore, DT can be performed in a single position and exposure and thus may be easier and faster than the PNS series, which requires multiple positions and exposures. With DT, by collecting a number of projection images at different angles using a digital detector, one can produce a limitless number of section images at random depths using a suitable reconstruction algorithm (1, 30). With better depth resolution and much less overlap of anatomic features than can be achieved using a PNS radiography series, DT might result in increased detection of paranasal sinus lesions.
Costs of non-contrast CT, DT, and PNS radiography series are approximately $200, $30, and $20, respectively, Korea (30). Therefore, DT is more expensive than the PNS radiography series, but still much cheaper than CT. Thus, DT may be suitable for follow-up examinations.
Our study showed that the overall diagnostic sensitivities of the DT technique for detection of sinusitis were higher than those of the PNS radiography series, although the mean scores of anatomic clearness for paranasal sinus were not significantly different in some PNSs (maxillary and ethmoid sinuses for observer 1, frontal sinuses for observer 2); because the PNS is composed of soft tissue and bones, the PNS XR series is sufficient for detection of anatomic conspicuity between bone and air. However, it is not sufficient for detection of soft tissue lesions.
The major limitation of DT is vulnerability to motion artifacts. The minimum required scan time of DT by commercially available machines is 3 seconds, and the usual scan time is about 10 seconds. During the scan, patients should fix their body and hold their breath. Therefore, a respiratory motion artifact can be critical in chest DT. In spite head and neck radiography is relatively tolerable to the respiratory motion, motion artifact can degrade image quality of DT in some patients who cannot fix their position especially in an aged person or a child (Fig. 4).
In our study, two observers who had different levels of experience with CT, radiography, and DT showed different characteristics in interpreting radiography and DT. Observer 1 was more specific and observer 2 was more sensitive. Despite the fact that the sensitivity and accuracy of the less experienced radiologist (observer 1) was lower for plain radiography, diagnostic performance for DT was not different than it was for the experienced radiologist. This suggests that DT is a more objective and easier method with which to diagnose sinusitis.
There were several limitations to our study. First, the number of patients enrolled in the study was rather small, and were cases involving the frontal sinus. Therefore, it is difficult to generalize our results. For example, the diagnostic performance values of DT were better than XR in observer 2; however, this difference was not significant. Second, we did not include skull lateral radiographs in the image analyses; inclusion of these images might have enhanced PNS XR sensitivity for sphenoid lesions. However, in our institute, standard plain radiography, such as Caldwell or Water's views, are used as the primary imaging methods. Thus, this clinical practice pattern may have reflected our routine daily work. Third, only OMU CT was used as the reference-standard method for the analysis; that method did not include additional evaluations, such as sinus endoscopy or any follow up clinical result. Therefore, some subclinical mucoperiosteal thickening on CT was regarded as sinusitis positive, and thus the sensitivities of the study were lower than expected.
In conclusion, patient radiation dose from low dose digital tomosynthesis was comparable to that of a PNS radiography series. Use of the DT technique is superior to use of radiography for detection of paranasal sinusitis and for acquiring anatomic details of the sphenoid sinus. DT can be considered as a good alternative to the XR PNS series for evaluation of paranasal sinusitis.
Figures and Tables
Fig. 1
Scores for anatomic conspicuity for radiography (XR) and digital tomosynthesis (DT) for demarcation of paranasal sinuses.
A. For observer 1, mean socres are 5.9 for XR and 6.8 for DT (p < 0.01). B. For observer 2, mean socres are 6.7 for XR and 8.1 for DT (p < 0.01).

Fig. 2
Images from 65-year-old man with fever.
A. Water's view radiograph shows subtle periosteal blurring of right maxillary sinuses. Both observers missed presence of sinusitis. B. Tomosynthesis image shows lobulating mucoperiosteal thickening in right mixillary sinus (arrow) and minimal and smooth thickening in left maxillary sinus (arrowheads). C. CT image confirms presence of mucoperiosteal thickening in both maxillary sinuses.

Fig. 3
Receiver operator characteristic curve (ROC curve) from radiography (XR) and digital tomosynthesis (DT) for detection of paranasal sinusitis.
A. For observer 1, areas under curve (Az) are 0.700 and 0.891, respectively, for XR and DT (p < 0.05). B. For observer 2, Azs are 0.813 and 0.924, respectively, for XR and DT (p < 0.05).

Fig. 4
Images from 68-year-old woman with cough.
A. Water's view radiograph shows subtle fluid filled left maxillary sinus. There is no motion artifact. B. Tomosynthesis image shows image blurring in right maxillary sinus (arrowheads) which mimic mucoperiosteal thickening. Note prominent motion artifact in mandible area. C. CT image confirms presence of left maxillary sinusitis. Note that right maxillary sinus is normal.
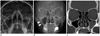
Table 1
Diagnostic Performance of Radiograph (XR) vs. Digital Tomosynthesis (DT) for Paranasal Sinusitis Per Patient Basis

References
1. Dobbins JT 3rd, Godfrey DJ. Digital x-ray tomosynthesis: current state of the art and clinical potential. Phys Med Biol. 2003. 48:R65–R106.
2. Grant DG. Tomosynthesis: a three-dimensional radiographic imaging technique. IEEE Trans Biomed Eng. 1972. 19:20–28.
3. Johnsson AA, Vikgren J, Svalkvist A, Zachrisson S, Flinck A, Boijsen M, et al. Overview of two years of clinical experience of chest tomosynthesis at Sahlgrenska University Hospital. Radiat Prot Dosimetry. 2010. 139:124–129.
4. Dobbins JT 3rd, McAdams HP. Chest tomosynthesis: technical principles and clinical update. Eur J Radiol. 2009. 72:244–251.
5. Dobbins JT 3rd, McAdams HP, Godfrey DJ, Li CM. Digital tomosynthesis of the chest. J Thorac Imaging. 2008. 23:86–92.
6. Vikgren J, Zachrisson S, Svalkvist A, Johnsson AA, Boijsen M, Flinck A, et al. Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: human observer study of clinical cases. Radiology. 2008. 249:1034–1041.
7. James TD, McAdams HP, Song JW, Li CM, Godfrey DJ, DeLong DM, et al. Digital tomosynthesis of the chest for lung nodule detection: interim sensitivity results from an ongoing NIH-sponsored trial. Med Phys. 2008. 35:2554–2557.
8. Sharifian H, Sharif Kashani S, Mazaher H, Abhari M. Assessment of the accuracy of paranasal sinuses limited CT scans in the diagnosis of Sinusitis. Iran J Radiol. 2006. 3:225–228.
9. Leibovitch I, Goldberg RA, Selva D. Paranasal sinus inflammation and non-specific orbital inflammatory syndrome: an uncommon association. Graefes Arch Clin Exp Ophthalmol. 2006. 244:1391–1397.
10. Roberts DN, Hampal S, East CA, Lloyd GA. The diagnosis of inflammatory sinonasal disease. J Laryngol Otol. 1995. 109:27–30.
11. Robinson KE. Roentgenographic manifestations of benign paranasal disease. Ear Nose Throat J. 1984. 63:144–149.
12. Dolan KD. Paranasal sinus radiology, Part 3A: sphenoidal sinus. Head Neck Surg. 1982. 5:164–176.
13. White PS, Cowan IA, Robertson MS. Limited CT scanning techniques of the paranasal sinuses. J Laryngol Otol. 1991. 105:20–23.
14. Wippold FJ 2nd, Levitt RG, Evens RG, Korenblat PE, Hodges FJ 3rd, Jost RG. Limited coronal CT: an alternative screening examination for sinonasal inflammatory disease. Allergy Proc. 1995. 16:165–169.
15. White PS, Robinson JM, Stewart IA, Doyle T. Computerized tomography mini-series: an alternative to standard paranasal sinus radiographs. Aust N Z J Surg. 1990. 60:25–29.
16. Sabol JM. A Monte Carlo estimation of effective dose in chest tomosynthesis. Med Phys. 2009. 36:5480–5487.
17. Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008. 248:995–1003.
18. Baldwin P. Digital breast tomosynthesis. Radiol Technol. 2009. 81:57M–74M.
19. Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol. 2010. 20:1545–1553.
20. Michell M, Wasan R, Whelehan P, Iqbal A, Lawinski C, Donaldson A, et al. Digital breast tomosynthesis: a comparison of the accuracy of digital breast tomosynthesis, two-dimensional digital mammography and two-dimensional screening mammography (film-screen). Breast Cancer Res. 2009. 11:Suppl 2. O1.
21. Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, et al. Digital breast tomosynthesis: observer performance study. AJR Am J Roentgenol. 2009. 193:586–591.
22. Schulz-Wendtland R, Fuchsjäger M, Wacker T, Hermann KP. Digital mammography: an update. Eur J Radiol. 2009. 72:258–265.
23. Duryea J, Dobbins JT 3rd, Lynch JA. Digital tomosynthesis of hand joints for arthritis assessment. Med Phys. 2003. 30:325–333.
24. Zachrisson S, Vikgren J, Svalkvist A, Johnsson AA, Boijsen M, Flinck A, et al. Effect of clinical experience of chest tomosynthesis on detection of pulmonary nodules. Acta Radiol. 2009. 50:884–891.
25. Tingberg A. X-ray tomosynthesis: a review of its use for breast and chest imaging. Radiat Prot Dosimetry. 2010. 139:100–107.
26. Lewin JM, Niklason L. Advanced applications of digital mammography: tomosynthesis and contrast-enhanced digital mammography. Semin Roentgenol. 2007. 42:243–252.
27. Bakic PR, Carton AK, Kontos D, Zhang C, Troxel AB, Maidment AD. Breast percent density: estimation on digital mammograms and central tomosynthesis projections. Radiology. 2009. 252:40–49.
28. Teertstra HJ, Loo CE, van den Bosch MA, van Tinteren H, Rutgers EJ, Muller SH, et al. Breast tomosynthesis in clinical practice: initial results. Eur Radiol. 2010. 20:16–24.
29. Zhang J, Wu QJ, Godfrey DJ, Fatunase T, Marks LB, Yin FF. Comparing digital tomosynthesis to cone-beam CT for position verification in patients undergoing partial breast irradiation. Int J Radiat Oncol Biol Phys. 2009. 73:952–957.
30. Kim EY, Chung MJ, Lee HY, Koh WJ, Jung HN, Lee KS. Pulmonary mycobacterial disease: diagnostic performance of low-dose digital tomosynthesis as compared with chest radiography. Radiology. 2010. 257:269–277.
31. Dobbins JT 3rd. Tomosynthesis imaging: at a translational crossroads. Med Phys. 2009. 36:1956–1967.
32. Taourel P, Merigeaud S, Aubert E, Millet I, Curros Doyon F, Lacroix J, et al. [Tomosynthesis: luxury or necessity?]. J Radiol. 2009. 90:1813–1821.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download