Abstract
We report on three cases of longstanding pulmonary tuberculosis patients with coronary to bronchial artery fistula (CBF) who presented with recurrent massive hemoptysis. The first and second patients died because of decreased functional pulmonary volume plus massive hemoptysis and cannulation failure of CBF due to hypovolemic vasospasm, respectively. When recurrent hemoptysis occurs despite successful embolization treatment, CBF should be considered as a potential bleeding source. Moreover, a coronary angiography should be performed, especially in patients with longstanding cardiopulmonary disease such as pulmonary tuberculosis.
Coronary to bronchial artery fistula (CBF) or anastomosis is congenital, and is considered an unusual finding (1-3). Most cases of CBF are asymptomatic (2-5). However, the coronary steal phenomenon can cause dyspnea and chest pain, and is currently treated with endovascular embolization or surgical ligation (3-6). Many reports of CBF focus on the effect of endovascular embolization with respect to the coronary steal phenomena or the possibility of embolic material regurgitation from the bronchial artery to the coronary artery during endovascular treatment (4-7).
Concurrent embolization of the bronchial artery and aberrant systemic arteries is an effective treatment for massive hemoptysis (8). For recurrent hemoptysis in patients with CBF, embolization of the bronchial artery via the CBF may be considered (6, 7). However, in most cases of recurrent massive hemoptysis treated with endovascular embolization, detection of CBF before the interventional procedure is very difficult (4, 6, 7).
We report three patients with recurrent massive hemoptysis and decreased cardiopulmonary reserve due to old pulmonary tuberculosis, two of whom died despite embolization treatment.
A 70-year-old male was treated with conservative management for a minor case of hemoptysis. However, massive hemoptysis (about 500 mL/24 hours) and hypoxemia occurred and the patient deteriorated. The patient had a history of pulmonary tuberculosis about 30 years prior, had suffered from congestive heart failure with arrhythmia for ten years, and had been treated for upper thoracic tuberculous spondylitis with posterior spinal fusion for the last 15 days.
A chest radiograph revealed severe destructive changes of both upper lobes, secondary emphysematous changes of both lower lobes and recent development of bilateral consolidations due to aspirated blood.
Embolization treatment was performed with a 5-Fr catheter and a coaxial 2.5-Fr microcatheter (Renegade; Boston Scientific, Watertown, MA) for both bronchial and other possible systemic arteries with polyvinyl alcohol (PVA) particles, 350-500 µm in diameter (Contour; Boston Scientific, Watertown, MA) and microcoils (Tornado embolization microcoil: Cook, Bloomington, IN). Two days later, the patient suffered massive hemoptysis. No evidence of recanalization of previously embolized arteries on angiography was found and additional embolization of both upper lobar pulmonary arteries was performed, because radiologically undetectable pulmonary artery bleeding was suspected. However, the patient continued to suffer from hemoptysis.
On the third trial of angiography, five hours after the second embolization, coronary angiography showed a CBF (proximal left circumflex arterial branch) from the left circumflex coronary artery to the left bronchial artery combined with a severe pulmonary artery shunt at the left upper lung. The CBF was embolized after superselection across the CBF to prevent embolization of the coronary artery (Fig. 1).
Hemoptysis was stopped immediately after the third embolization but the patient died 12 hours later due to further deterioration of hypoxemia despite intensive ventilator care.
A 57-year-old male arrived at the emergency room due to cardiac arrest. He had a history of diabetes and pneumonia six and two years prior, respectively, and underwent embolization treatment for hemoptysis five days prior and was discharged one day earlier. He was diagnosed with pulmonary and endobronchial tuberculosis and was prescribed medications for tuberculosis.
About one hour before arriving at the emergency room, about 700 mL of hemoptysis occurred. For the first angiography performed five days prior, the right interocostobronchial artery to the left lower lung, aberrant bronchial arteries from the left internal mammary artery supplying the left upper lung, and several left posterior intercostal arteries supplying the left lower lung were successfully embolized with PVA particles.
Computed tomography (CT) showed no enlarged bronchial arteries, and angiography of the descending thoracic aorta and left subclavian artery showed no systemic hypervascularization in either lung. Both coronary angiographies showed a CBF (sinoatrial nodal artery) from the right coronary artery coursing along the left main bronchus (Fig. 2). However, cannulation and embolization of CBF failed due to spastic changes of CBF and tachycardia. Bleeding through the endotracheal tube and hypoxemia persisted despite intensive care resulting in death two days after embolization.
A 68-year-old female experienced four episodes of hemoptysis (more than 400 mL/48 hours). She had a history of pulmonary tuberculosis that was diagnosed about 50 years prior and had underwent embolization of both bronchial arteries and multiple systemic collaterals due to recurrent hemoptysis (seven times) over the five years prior to presentation. Serial chest radiographs and CT showed severe destructive changes with bronchiectasis and cavitary lesions at the left lung.
Because bronchial or systemic arteries causing hemoptysis were not found, right and left coronary angiographies were performed. The coronary angiography revealed two CBFs from both coronary arteries (sinoatrial nodal artery from right coronary artery and distal left circumflex arterial branch) to the left upper and lower lung as well as a massive pulmonary artery shunt (Fig. 3). After careful selection of CBFs as far from the coronary arterial branches as possible with a microcatheter, an embolization was performed with PVA particles and small amounts of Gelfoam particles, resulting in no visible residual hypervascularization or pulmonary artery shunt. The patient was asymptomatic without hemoptysis, but abnormal electrocardiography (EKG) (unusual P axis and short PR, probably junctional tachycardia) and increased cardiac enzyme (serum creatine kinase 375 IU/L, normal range 22-269; creatine kinase-MB 33.0 ng/mL, normal range 0.0-3.4; troponin-I 11.04 ng/mL, normal range 0.0-0.3) were noted the next day. Echocardiography showed normal left ventricular systolic function and EKG was normalized on the sixth day post-embolization. The patient was discharged without any symptoms.
Coronary to bronchial artery fistula is present at birth and remains closed and asymptomatic in most of the population. However, this anastomosis may become enlarged and functional in various cardiovascular and chronic pulmonary diseases with an incidence of about 0.6%. Localized bronchiectasis is the most common condition associated with CBF; however, our three cases the CBFs were combined with pulmonary tuberculosis (2-4, 6).
Although chest pain is the most frequent symptom of CBF, massive hemoptysis can also occur (5, 6, 8, 9). The detection of CBF as a cause of hemoptysis is challenging, because CBF is a rare cause of hemoptysis (5, 6). In addition, CBF is seldom detected prior to embolization treatment for hemoptysis if coronary angiography is not performed (6). For detection of coronary and pulmonary artery abnormalities in patients with hemoptysis, special coronary or pulmonary CT angiography protocol is required (2, 10).
There are reports of successful embolization of bronchial arteries originating from a coronary artery for control of hemoptysis; however, unlike our cases, the presence of CBF was fortunately suspected on CT coronary angiography prior to the embolization procedure (5, 9).
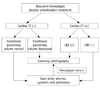
Delayed diagnosis and treatment of arteries involved in systemic hypervascularization of the lung, including bronchial arteries, might lead to catastrophic consequences in patients with hemoptysis. Furthermore, massive hemoptysis may be more likely to be fatal in patients with reduced cardiopulmonary reserve, as in our cases (8, 10). Our patients had decreased functional pulmonary parenchymal volume due to pulmonary tuberculosis, and therefore any insult could have been fatal (Fig. 4).
Although symptomatic CBF is extremely rare, suspicion of the presence and detection of CBF is crucial to avoid serious complications during interventional procedures (4, 7, 9). Further, proper knowledge of CBF may help clinicians to treat patients with hemoptysis and prevent procedure-related complications.
In conclusion, when recurrent hemoptysis occurs despite successful embolization of possible systemic arteries, a coronary angiography should be performed, especially in patients with reduced cardiopulmonary reserve and longstanding cardiopulmonary disease.
Figures and Tables
Fig. 1
Angiographies of both bronchial arteries and coronary-to-bronchial artery fistula in 70-year-old male, case 1.
A, B. Both bronchial arteries are enlarged and embolized on initial embolization treatments. C. Coronary to bronchial artery fistula (arrow, proximal left circumflex arterial branch) to left upper lung with severe pulmonary artery shunt (not shown) is noted on left coronary angiography.
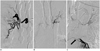
Fig. 2
Chest CT and angiography of right coronary artery in 57-year-old male, case 2.
Chest CT images prior to first (A) and second (B) embolization treatments. Right lung lesions were improving but left lung lesions changed into cavitary lesions. Right coronary angiography (C, D) shows coronary to bronchial artery fistula with spastic change from proximal right coronary artery running along superior and inferior walls of left main bronchus (arrows).
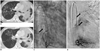
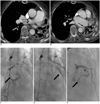
Fig. 3
Chest CT and angiographies of both coronary arteries in 68-year-old female, case 3.
A, B. Chest CT images prior to embolization treatment. Small tortuous coronary to bronchial artery fistula is suspected to be traversing right main pulmonary artery (thin arrows, A), and enlarged left and circumflex coronary arteries are seen (thin arrow, B). C, D. Early and late phases of right coronary angiography show enlarged coronary to bronchial artery fistula from proximal right coronary artery and massive pulmonary artery shunt (thick arrows). E. Left coronary angiography shows another enlarged coronary to bronchial artery fistula (thick arrow) that showed severe pulmonary artery shunt at left lower lung (not shown).
References
1. Von Haller A. First lines of physiology. 1803. 1st ed. Troy, OH: Penniman;35.
2. Lee ST, Kim SY, Hur G, Hwang YJ, Kim YH, Seo JW, et al. Coronary-to-bronchial artery fistula: demonstration by 64-multidetector computed tomography with retrospective electrocardiogram-gated reconstructions. J Comput Assist Tomogr. 2008. 32:444–447.
3. Matsunaga N, Hayashi K, Sakamoto I, Ogawa Y, Matsuoka Y, Imamura T, et al. Coronary-to-pulmonary artery shunts via the bronchial artery: analysis of cineangiographic studies. Radiology. 1993. 186:877–882.
4. Jim MH, Lee SW, Lam L. Localized bronchiectasis is a definite association of coronaro-bronchial artery fistula. J Invasive Cardiol. 2003. 15:554–556.
5. Cho J, Shin T, Jun K, Ryoo J, Choi H, Choi B, et al. Transcatheter embolization of bronchial artery arising from left circumflex coronary artery in a patient with massive hemoptysis. Cardiovasc Intervent Radiol. 2010. 33:169–172.
6. Miyazono N, Inoue H, Hori A, Kanetsuki I, Shimada J, Nakajo M. Visualization of left bronchial-to-coronary artery communication after distal bronchial artery embolization for bronchiectasis. Cardiovasc Intervent Radiol. 1994. 17:36–37.
7. Peynircioglu B, Ergun O, Hazirolan T, Cil BE, Aytemir K. Bronchial to coronary artery fistulas: an important sign of silent coronary artery disease and potential complication during bronchial artery embolization. Acta Radiol. 2007. 48:171–172.
8. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002. 22:1395–1409.
9. Jarry G, Bruaire JP, Commeau P, Hermida JS, Leborgne L, Auquier MA, et al. Coronary-to-bronchial artery communication: report of two patients successfully treated by embolization. Cardiovasc Intervent Radiol. 1999. 22:251–254.
10. Shin S, Shin TB, Choi H, Choi JS, Kim YH, Kim CW, et al. Peripheral pulmonary arterial pseudoaneurysms: therapeutic implications of endovascular treatment and angiographic classifications. Radiology. 2010. 256:656–664.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download