Abstract
In keeping with the increasing utilization of CT examinations, the greater concern about radiation hazards from examinations has been addressed. In this regard, CT radiation dose optimization has been given a great deal of attention by radiologists, referring physicians, technologists, and physicists. Dose-saving strategies are continuously evolving in terms of imaging techniques as well as dose management. Consequently, regular updates of this issue are necessary especially for radiologists who play a pivotal role in this activity. This review article will provide an update on how we can optimize CT dose in order to maximize the benefit-to-risk ratio of this clinically useful diagnostic imaging method.
Since the introduction of the multi-section CT, the speed and z-axis coverage of CT scanning have dramatically increased. As a result, the clinical utility has considerably increased in our practice not only in general applications, but also in newer applications such as cardiac CT (1, 2) and dual energy CT (3). As CT utilization increases, the concern about radiation hazards from CT also increases (4). In fact, the worldwide average annual per-capita effective dose from medical procedures has approximately doubled in the past 10-15 years (5). A study (5) has also found an uneven distribution of medical radiation exposure, which is greater in highly developed countries. For example, the 2006 United States data showed that medical imaging contributed to approximately half (3.0 mSv) of the total radiation dose (5.6 mSv) (5, 6). The greatest contributor to medical radiation exposure is CT. In the United States, the number of CT scans is increasing by approximately 10% per year (5, 6). In South Korea, the increasing rate is even steeper, approximately 11-31% per year (7).
In conjunction with the increasing concerns about potential CT radiation hazards, various CT dose-saving strategies have been developed (8, 9). Thus, the benefit-risk ratio of CT examinations can be maximized with optimized CT imaging techniques using these strategies. Although there are several uncertainties in quantifying life-time risks from CT examinations, per-capita cumulative CT radiation dose should be minimized particularly in the younger population because they have unequivocally higher radiosensitivity and longer life expectancy than the older population. In this article, currently available CT dose-saving strategies will be reviewed, which will ultimately facilitate our rational use of CT.
For dose optimization, understanding CT dose parameters including tube potential, tube current, pitch, weighted CT dose index (CTDIw), volume CT dose index (CTDIvol), dose-length product (DLP), and effective dose is a prerequisite. The definition of each parameter and its effects on radiation dose are summarized in Table 1. The ultimate goal of dose optimization is to minimize radiation dose for obtaining diagnostic quality of CT images. Therefore, we need to determine how to obtain diagnostic quality of CT images for dose optimization. The four fundamental elements determining CT image quality consist of image noise, image contrast, spatial resolution, and artifacts (Table 2). In principle, radiation dose is inversely proportional to the square of the image noise. Image contrast is significantly augmented by the use of a contrast agent and is influenced by tube potential in some materials with high atomic numbers such as iodine due to the different photoelectric interactions (Fig. 1). The required image quality of CT differs somewhat among different diagnostic tasks. Consequently, the required CT radiation dose is also fairly diverse and thus should be tailored according to clinical indications. For example, very low dose CT can be used for the identification of high-contrast lesions, such as urinary stones, colonic polyps (virtual colonography), or lung nodules.
To be compliant with the so-called "as low as reasonably achievable (ALARA)" principle, it is imperative to justify CT examinations beforehand. In this respect, radiologists should play an important advisory role in this decision with referring clinicians. When equal or greater diagnostic yields are expected, CT should be replaced by alternative imaging modalities with no or less ionizing radiation, such as sonography, magnetic resonance (MR) imaging, or radionuclide voiding cystography. On the other hand, radiologists should make every effort to reduce the radiation dose of CT examinations while maintaining diagnostic quality when CT is indicated (8-10). For example, minimizing the scan range of CT examinations as required is a straightforward way to achieve this goal. For multi-phase CT protocols, the number of repeated scannings should be minimized and precontrast scanning should be used only when diagnostic information on precontrast CT images is not obtainable from postcontrast scanning. Because of the substantial radiation dose of perfusion CT, its clinical indication and imaging protocol should be carefully determined (11). In the following sections, other useful CT dose-saving strategies are described with recent updates. A checklist for CT dose optimization is described in Table 3.
Body size-adapted CT protocol is a fundamental part of CT dose optimization because the minimal radiation dose required for diagnostic image quality would be varied even at the same diagnostic task depending on body size and habitus. Then, the optimal tube voltage and current should be determined for the adapted radiation dose. One of the common misconceptions is that lowering the tube voltage at the same tube current is a good strategy for low dose CT. Actually, a higher tube current should be used at a lower tube potential to compensate for the increased image noise (12, 13). Another misconception is that tube current modulation can automatically adapt the CT radiation dose to different body sizes and anatomic regions and therefore allows a constant image quality. However, this is erroneous and a user-defined target image quality considering patient size and diagnostic task should be determined for each anatomic region and each tube voltage.
Various patient size parameters have been used for body size-adapted CT protocols for both children and adults (13, 14). Among the parameters, body weight or body mass index has been traditionally applied to body size-adapted CT protocols due to its easy applicability (13, 14). Many investigators however have found that cross-sectional dimensions are better adapted to body habitus than the traditional parameters, i.e. body weight or body mass index (15-19). An attenuation-based parameter using a scout has been used in other studies (20, 21). The image noise measured on calcium scoring images (22) or a timing bolus image (23) was used to obtain uniform image quality of cardiac CT. Nevertheless, such parameters other than body weight or body mass index have not been commonly used for body size-adapted CT protocols mainly because of a difficulty in clinical implementation. Recently, a practical pediatric chest CT protocol based on cross-sectional area and mean attenuation of the body was developed and provided less noise variation irrespective of body habitus (24). In a study (25), CTDIvol was used to represent an optimal radiation dose for an individual, and other factors determining image noise such as section thickness, image reconstruction algorithm, and tube voltage were also taken into consideration to establish the optimal radiation dose. A CT protocol derived from a best-fit equation is generally preferred to a classic CT dose table or chart for more accurate dose adaptation and better compliance.
Tube current modulation greatly contributes to CT dose optimization by reducing the CT dose according to body size, shape, and attenuation without degrading image quality. The tube current may be adjusted in the x-y plane (angular mode), the z-axis, or a combination of both. CT dose reduction achieved by tube current modulation has been reported to be up to 26-50% in children and adults (25-27). We need to understand the principles of different tube current modulation techniques for their proper use (28). In addition, a patient should be positioned at the CT isocenter to avoid faulty modulation of the tube current. Other factors influencing tube current modulation include tube voltage, maximum tube current, scan speed, and scan direction (29-31). Indeed, the fact that the tube current is frequently saturated to its maximum level at a lower tube voltage, faster scan speed, or a combination of both has seldom been recognized (30, 31). With thicker or denser body parts, the tube current saturation occurs earlier. Since image quality is subject to deterioration resulting from the increased image noise at regions scanned at a saturated tube current, the use of a higher tube voltage, slower scan speed (either slower gantry rotation time or a lower pitch), or a combination of both should be considered as a remedy for this potential pitfall. As previously mentioned, the target image quality index for tube current modulation, which differs according to patient size, each anatomic region, individual diagnostic task, and tube voltage, should be set currently by an operator. In this regard, we should be aware that tube current modulation is not truly automatic. The experience on how we can determine this target image quality index for tube current modulation is very limited (24, 32, 33).
Optimal tube voltage should be determined for patient size and each type of CT examination to achieve an optimal tradeoff between contrast, noise, artifacts, and scanning speed (13, 34, 35). Concrete knowledge on CT physics and the diagnostic purposes of CT examination is mandatory for this task. The importance of optimal tube voltage has been recently emphasized for CT dose optimization in order to maximize the clinical benefits of CT examination at a low radiation dose and in order to determine the most dose-efficient tube voltage. Based on CT physics, the iodine contrast, image noise, and iodine contrast-to-noise ratio (CNR) show different behaviors at different tube voltages and different phantom sizes: increasing iodine contrast at lower tube voltages, that is, decreasing for larger phantoms; almost identical noise level for a 10-cm phantom and a dramatic increase in noise level for a 40-cm phantom; markedly increasing iodine CNR at lower tube voltages for a 10-cm phantom; and minimally increasing iodine CNR at lower tube voltages for a 40-cm phantom (34). In regard to the diagnostic task, radiologists should determine the degree of importance of iodine CNR or image noise for a particular type of CT examination. In general, iodine CNR is more important in contrast-enhanced examinations, while image noise is more important in precontrast examinations or in detecting low-contrast lesions and is less important in detecting high-contrast lesions. In contrast to the benefits of lower tube voltages to contrast-enhanced CT, the potential benefits of higher tube voltages to CT exams requiring lower image noise have not been thoroughly investigated. For instance, image noise should be sufficiently low to increase low-contrast detectability. The reduction of image noise can be achieved not only by using an adaptive noise reduction filter (36) or sliding-thin-slab averaging algorithm (37), but also by simply using a higher tube voltage. Likewise, a higher noise at lower tube voltages may adversely affect the assessment of ground-glass opacity in the lungs (38). Higher tube voltages are also commonly used in an unenhanced brain CT requiring lower noise in assessing low-contrast intracranial structures (13, 39, 40). Furthermore, a higher tube voltage may produce less severe artifacts from metallic objects or thick bones such as the skull base than a lower tube voltage. A general strategy for selecting optimal tube voltage at different phantom sizes and different noise constraints (reflecting different diagnostic tasks) was recently proposed (41, 42).
Several CT scan modes are available for clinical CT examinations, including spiral scanning with or without ECG synchronization, sequential scanning with or without ECG synchronization, and dual energy spiral scanning. Dose issues specific to each scan mode are described in the following section.
Overbeaming is the waste dose beyond the edge of the detector rows of a multi-section CT (Fig. 2). The magnitude of overbeaming is inversely proportional to the number of detector rows. Therefore, its contribution to unnecessary radiation exposure to patients has reduced with modern multi-section CT systems. To acquire spiral scanning, a CT system needs at least half a rotation beyond the planned scan length in order to reconstruct the first and the last images (Fig. 2). This unnecessary radiation exposure outside the planned scan length is called as overranging. Overranging is proportional to beam collimation, reconstructed slice width, and pitch, while it is irrespective of a planned scan length (43). The contribution of overranging to the total CT dose is therefore considerably higher for CT examinations with shorter scan ranges such as pediatric CT and cardiac CT (44). Fortunately, adaptive section collimation technology was recently developed to eliminate overranging during spiral scanning (45) (Fig. 2). This useful collimation technology is currently available only in some of the CT models.
Cardiac CT with retrospective ECG gating was renowned for delivering higher radiation exposure to patients. The radiation dose of cardiac CT is now comparable to or even lower than that of chest CT due to innovative dose-reducing techniques (46). The relatively higher radiation dose, approximately 15 mSv on average, of retrospectively ECG-gated spiral scanning used for cardiac or coronary CT angiography is mainly attributed to the low pitch factor. For example, 0.2. ECG-based tube current modulation, in which 20% or 4% of the nominal value is used outside a target cardiac phase, can reduce the radiation dose of retrospectively ECG-gated spiral scanning to approximately 10 mSv or 6 mSv, respectively (2, 47). The use of heart rate-dependent pitch values can additionally reduce the radiation dose of cardiac CT at higher heart rates (48).
Prospectively ECG-triggered sequential (or step-and-shoot) scanning can further reduce the radiation dose of cardiac CT in the range of 1-4 mSv (2, 46, 49). This sequential scanning can be used in other body regions. However, potential pitfalls related to this scan mode, including prolonged scan time and stair-step artifacts due to different contrast enhancement or motion misregistration, should be carefully considered prior to the examination (2).
High-pitch dual source spiral scanning with or without ECG triggering is the most recent advance in CT imaging techniques. With this scan mode, pitch can be increased to 3.0-3.4, which results in a substantial reduction of radiation dose of cardiac CT, by approximately 1 mSv (46, 50). This high-pitch spiral scanning considerably decreases not only motion artifacts but also the requirement of sedation in free-breathing patients. Hence, the scan mode is regarded as very useful in pediatric patients and uncooperative adult patients (51). However, it should be noted that overranging increases with this scan mode due to a combination of high pitch and longer collimation. Moreover, adaptive section collimation technology to protect this overranging is not available for this scan mode.
Dual energy CT has expanded clinical applications of CT examinations, along with cardiac CT. Dual energy scanning can be performed with either two X-ray sources, kVp switching of one X-ray source, or dual-layer ("sandwich") detectors (3, 52, 53). Each method has different radiation dose profiles. Dual energy scanning using a dual-source CT system is almost dose-equivalent to single energy scanning (3, 54-59). In contrast, the radiation dose of dual energy scanning using a single-source system with rapid kVp switching is currently higher (e.g. 8 mSv for dual energy chest CT) than that of dual-source dual energy scanning or single energy scanning (52). A recent study (60) showed that the use of additional tin filtration in the high-energy X-ray beam of a dual-source CT system provided several benefits for dual energy CT applications, including a similar or lower radiation dose compared with the conventional single energy CT, increased dual-energy contrast, and improved image quality of dual-energy material-specific (e.g. virtual noncontrast) images. Moreover, the virtual noncontrast imaging of dual energy CT has a potential to reduce the radiation dose by omitting precontrast scanning (61).
The use of noise-reducing image reconstruction algorithms may have a potential to reduce the CT radiation dose. However, conventional noise-reduction filters decrease image noise but simultaneously decrease lesion contrast and conspicuity (62) (Fig. 3). This trade-off actually limits the dose-saving potential of these noise-reduction filters. Recently, decoupling between image noise and spatial resolution has been performed in noise-reducing image reconstruction algorithms using iterative reconstruction (63, 64). Consequently, a 40-50% dose reduction can be achieved by means of iterative reconstruction algorithms without degrading image quality. Iterative reconstruction algorithms are continuously improving in terms of image quality and reconstruction speed. We may anticipate that conventional filtered back projection will eventually be replaced by an iterative reconstruction algorithm and that high image quality will be achieved at a very low dose in the near future.
The use of beam-shaping filters (e.g. bowtie filters) can reduce the absorbed radiation in the periphery of the scanned body, which is particularly useful in pediatric CT and cardiac CT (33). In-plane shielding may be used for reducing radiation exposure to radiation-sensitive organs, such as the breast, thyroid, and eye lens by 20-50% (64, 65). Shields are however associated with greater image noise, artifactually increased attenuation values, and streak artifacts (65). When used with tube current modulation, a greater dose reduction is achieved by placing the shield after obtaining a scout image (66). Shields are not commonly used partly due to their cost and for sanitary reasons. On the other hand, organ-based tube current modulation was recently developed and a 27-50% dose reduction to the anterior radiosensitive organs for head and chest CT scans could be achieved without increasing image noise and without the use of shields (67). For contrast-enhanced CT examinations, higher contrast enhancement can be used for a dose-saving technique by compensating for the higher image noise resulting from a low radiation dose (68).
The actual risks of radiation exposure from low-dose diagnostic imaging are considerably uncertain (6). However, many investigators believe that low levels of ionizing radiation in the range of 5-125 mSv have a very small but statistically significant increase in cancer risk. Several factors predominantly influencing cancer risk from radiation exposure should be carefully considered in establishing CT protocols. These include genetic susceptibility, age at exposure, and sex. The estimation of CT dose helps to provide some direction in terms of CT dose optimization. The estimation itself also may increase the awareness of the necessity of CT dose optimization. For dose estimation, the effective dose is generally used because the concept can indicate the amount of whole-body average irradiation resulting from the partial-body irradiation of diagnostic imaging and can be used to compare radiation doses between different procedures. Two methods are used to calculate the dose estimates: one method is the Monte Carlo simulation by using standard mathematical phantoms, for which CT dosimetry calculators such as ImPACT (UK National Health Service CT Evaluation Centre, London, England) and CT Expo (Medizinische Hochschule, Hannover, Germany) are commercially available; the other method is a DLP-based method in which CT dose estimates are calculated by multiplying a DLP value and an appropriate conversion factor. The former also can provide estimates of the lifetime attributable risk of cancer. Tissue weighting factors were recently updated in the International Commission on Radiological Protection (ICRP) publication 103. Major changes involve the gonads (0.20 → 0.08) and breast (0.05 → 0.12), which culminates in a decrease in dose estimates of pelvic CT and abdominopelvic CT and an increase in chest CT and cardiac CT. Accordingly, conversion factors for the DLP-based method should be updated (69) (Table 4). We should take notice that the effective dose of the same CT examination is subject to being changed according to the conversion factors used for calculating DLP-based CT dosimetry. Therefore, it is highly recommended that CTDIvol and DLP values be described along with CT dose estimates.
Data on cumulative CT dose estimates can be used to further tailor and eventually improve CT dose optimization strategies or guidelines. This type of investigation conducted in patients who have CT examinations at a young age will be of a great value, as pointed out in the report on the Biologic Effects of Ionizing Radiation (BEIR) VII (70). In fact, such studies based on actual individual CT dosimetry are ongoing in South Korea and other countries.
Diagnostic reference levels (DRLs) of various types of CT examinations, defined as the third quartile of the collected dose data, offer good reference values in CT dose optimization. This activity identifies high dose practices and encourages the imagers to determine the causes and solutions to using a relatively higher dose, which results in modification of such practices to lower the dose. The DRLs have shown a tendency to gradually decrease due to recent developments in dose-reducing techniques and increased awareness of radiation dose issues, but they still show wide variations (71-75). The DRLs of head, chest, and abdominal CT examinations in adults from different countries are described in Table 5.
In this review article, the essential requirements and latest updates of CT dose optimization for radiologists are described. Because strategies for CT dose optimization and estimation of radiation risks are constantly evolving and being updated, educational efforts including this review article should also be continuous and regularly updated. From this study, radiologists will undoubtedly be ready for exploring the clinical benefits of CT.
Figures and Tables
Fig. 1
Axial contrast-enhanced chest CT images using dual-source CT system with different energy levels (A: 80 kVp, B: mixed with composition ratio of 0.4, C: 140 kVp with tin filter).
Degree of contrast enhancement is higher at 80 kVp (A) than at 140 kVp with tin filter (C) as result of different X-ray linear attenuation coefficients between iodine and water. Images (A, C) reconstructed from single X-ray source appear to be noisier than mixed image (B) because of difference in radiation dose by factor of approximately two.

Fig. 2
Diagram illustrating overbeaming, overranging, and adaptive section collimation technology during spiral scanning.
Fig. 3
Volume-rendered cardiac CT images seen from feet.
Compared with image using standard reconstruction algorithm (A), image using conventional noise-reducing reconstruction filter (B) shows degraded anatomic details of small peripheral pulmonary vessels.
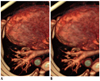
Fig. 4
Graph showing difference in radiation dose between peak dose and CT dose index (modified from reference [60]). Dose from CT scanning without table increment is overestimated by factor of two or more with CT dose index (CTDI) values in comparison with point dose values.

Table 4
Age- and Sex-Specific Conversion Factors for Dose-Length Product-Based CT Dosimetry Based on International Commission on Radiological Protection (ICRP) Publication 103, Modified from Reference (54)

Note.- For proper CT dose estimation in gray cells (i.e. body CT examinations in adults), dose-length product values should be derived from 32-cm diameter CT dosimetry phantom. Dose-length product values should be derived from 16-cm diameter CT dosimetry phantom in other situations (i.e. head and neck CT examinations in adults; all pediatric CT examinations).
References
1. Mahnken AH, Muhlenbruch G, Gunther RW, Wildberger JE. Cardiac CT: coronary arteries and beyond. Eur Radiol. 2007. 17:994–1008.
2. Goo HW. State-of-the-art CT imaging techniques for congenital heart disease. Korean J Radiol. 2010. 11:4–18.
3. Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007. 17:1510–1517.
4. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007. 357:2277–2284.
5. Mettler FA Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009. 253:520–531.
6. Hricak H, Brenner DJ, Adelstein SJ, Frush DP, Hall EJ, Howell RW, et al. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011. 258:889–905.
7. Assessments on the 2006 state of CT claims reported by the Korea Health Insurance Review and Assessment Service. Accessed on November 3, 2011. Available from URL: http://biz.hira.or.kr/ICSFiles/afieldfile/2008/01/21/2006_CT.pdf.
8. Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, et al. Strategies for CT radiation dose optimization. Radiology. 2004. 230:619–628.
9. Goo HW. Pediatric CT: understanding of radiation dose and optimization of imaging techniques. J Korean Radiol Soc. 2005. 52:1–5.
10. Singh S, Kalra MK, Moore MA, Shailam R, Liu B, Toth TL, et al. Dose reduction and compliance with pediatric CT protocols adapted to patient size, clinical indication, and number of prior studies. Radiology. 2009. 252:200–208.
11. Ketelsen D, Horger M, Buchgeister M, Fenchel M, Thomas C, Boehringer N, et al. Estimation of radiation exposure of 128-slice 4D-perfusion CT for the assessment of tumor vascularity. Korean J Radiol. 2010. 11:547–552.
12. Park EA, Lee W, Kang JH, Yin YH, Chung JW, Park JH. The image quality and radiation dose of 100-kVp versus 120-kVp ECG-gated 16-slice CT coronary angiography. Korean J Radiol. 2009. 10:235–243.
13. Yang DH, Goo HW. Pediatric 16-slice CT protocol: radiation dose and image quality. J Korean Radiol Soc. 2008. 59:333–347.
14. Tatsugami F, Husmann L, Herzog BA, Burkhard N, Valenta I, Gaemperli O, et al. Evaluation of a body mass index-adapted protocol for low-dose 64-MDCT coronary angiography with prospective ECG triggering. AJR Am J Roentgenol. 2009. 192:635–638.
15. Starck G, Lonn L, Cederblad A, Forssell-Aronsson E, Sjostrom L, Alpsten M. A method to obtain the same levels of CT image noise for patients of various sizes, to minimize radiation dose. Br J Radiol. 2002. 75:140–150.
16. Boone JM, Geraghty EM, Seibert JA, Wootton-Gorges SL. Dose reduction in pediatric CT: a rational approach. Radiology. 2003. 228:352–360.
17. Kalra MK, Maher MM, Prasad SR, Hayat MS, Blake MA, Varghese J, et al. Correlation of patient weight and cross-sectional dimensions with subjective image quality at standard dose abdominal CT. Korean J Radiol. 2003. 4:234–238.
18. Jung YY, Goo HW. The optimal parameter for radiation dose in pediatric low dose abdominal CT: cross-sectional dimensions versus body weight. J Korean Radiol Soc. 2008. 58:169–175.
19. Nyman U, Ahl TL, Kristiansson M, Nilsson L, Wettemark S. Patient-circumference-adapted dose regulation in body computed tomography. A practical and flexible formula. Acta Radiol. 2005. 46:396–340.
20. Reid J, Gamberoni J, Dong F, Davros W. Optimization of kVp and mAs for pediatric low-dose simulated abdominal CT: is it best to base parameter selection on object circumference? AJR Am J Roentgenol. 2010. 195:1015–1020.
21. Menke J. Comparison of different body size parameters for individual dose adaptation in body CT of adults. Radiology. 2005. 236:565–571.
22. Hur G, Hong SW, Kim SY, Kim YH, Hwang YJ, Lee WR, et al. Uniform image quality achieved by tube current modulation using SD of attenuation in coronary CT angiography. AJR Am J Roentgenol. 2007. 189:188–196.
23. Qi W, Li J, Du X. Method for automatic tube current selection for obtaining a consistent image quality and dose optimization in a cardiac multidetector CT. Korean J Radiol. 2009. 10:568–574.
24. Irie T, Inoue H. Individual modulation of the tube current-seconds to achieve similar levels of image noise in contrast-enhanced abdominal CT. AJR Am J Roentgenol. 2005. 184:1514–1518.
25. Goo HW. Individualized volume CT dose index determined by cross-sectional area and mean density of the body to achieve uniform image noise of contrast-enhanced pediatric chest CT obtained at variable kV levels and with combined tube current modulation. Pediatr Radiol. 2011. 41:839–847.
26. Greess H, Wolf H, Baum U, Lell M, Pirkl M, Kalender W, et al. Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol. 2000. 10:391–394.
27. Goo HW, Suh DS. Tube current reduction in pediatric non-ECG-gated heart CT by combined tube current modulation. Pediatr Radiol. 2006. 36:344–351.
28. Rizzo S, Kalra M, Schmidt B, Dalal T, Suess C, Flohr T, et al. Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR Am J Roentgenol. 2006. 186:673–679.
29. Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, et al. Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics. 2008. 28:1451–1459.
30. Goo HW, Suh DS. The influences of tube voltage and scan direction on combined tube current modulation: a phantom study. Pediatr Radiol. 2006. 36:833–840.
31. Israel GM, Herlihy S, Rubinowitz AN, Cornfeld D, Brink J. Does a combination of dose modulation with fast gantry rotation time limit CT image quality? AJR Am J Roentgenol. 2008. 191:140–144.
32. Prakash P, Kalra MK, Gilman MD, Shepard JA, Digumarthy SR. Is weight-based adjustment of automatic exposure control necessary for the reduction of chest CT radiation dose? Korean J Radiol. 2010. 11:46–53.
33. Goo HW. Cardiac MDCT in children: CT technology overview and interpretation. Radiol Clin North Am. 2011. 49:997–1010.
34. Yu L, Bruesewitz MR, Thomas KB, Fletcher JG, Kofler JM, McCollough CH. Optimal tube potential for radiation dose reduction in pediatric CT: principles, clinical implementations, and pitfalls. Radiographics. 2011. 31:835–848.
35. Kalender WA, Buchenau S, Deak P, Kellermeier M, Langner O, van Straten M, et al. Technical approaches to the optimisation of CT. Phys Med. 2008. 24:71–79.
36. Funama Y, Awai K, Miyazaki O, Nakayama Y, Goto T, Omi Y, et al. Improvement of low-contrast detectability in low-dose hepatic multidetector computed tomography using a novel adaptive filter: evaluation with a computer-simulated liver including tumors. Invest Radiol. 2006. 41:1–7.
37. von Falck C, Hartung A, Berndzen F, King B, Galanski M, Shin HO. Optimization of low-contrast detectability in thin-collimated modern multidetector CT using an interactive sliding-thin-slab averaging algorithm. Invest Radiol. 2008. 43:229–235.
38. Bankier AA, Tack D. Dose reduction strategies for thoracic multidetector computed tomography: background, current issues, and recommendations. J Thorac Imaging. 2010. 25:278–288.
39. Cohnen M, Fischer H, Hamacher J, Lins E, Kotter R, Modder U. CT of the head by use of reduced current and kilovoltage: relationship between image quality and dose reduction. AJNR Am J Neuroradiol. 2000. 21:1654–1660.
40. Mullins ME, Lev MH, Bove P, O'Reilly CE, Saini S, Rhea JT, et al. Comparison of image quality between conventional and low-dose nonenhanced head CT. AJNR Am J Neuroradiol. 2004. 25:533–538.
41. Kalender WA, Deak P, Kellermeier M, van Straten M, Vollmar SV. Application- and patient size-dependent optimization of x-ray spectra for CT. Med Phys. 2009. 36:993–1007.
42. Yu L, Li H, Fletcher JG, McCollough CH. Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Med Phys. 2010. 37:234–243.
43. van der Molen AJ, Geleijns J. Overranging in multisection CT: quantification and relative contribution to dose--comparison of four 16-section CT scanners. Radiology. 2007. 242:208–216.
44. Tzedakis A, Damilakis J, Perisinakis K, Karantanas A, Karabekios S, Gourtsoyiannis N. Influence of z overscanning on normalized effective doses calculated for pediatric patients undergoing multidetector CT examinations. Med Phys. 2007. 34:1163–1175.
45. Deak PD, Langner O, Lell M, Kalender WA. Effects of adaptive section collimation on patient radiation dose in multisection spiral CT. Radiology. 2009. 252:140–147.
46. Alkadhi H, Leschka S. Radiation dose of cardiac computed tomography - what has been achieved and what needs to be done. Eur Radiol. 2011. 21:505–509.
47. Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002. 12:1081–1086.
48. McCollough CH, Primak AN, Saba O, Bruder H, Stierstorfer K, Raupach R, et al. Dose performance of a 64-channel dual-source CT scanner. Radiology. 2007. 243:775–784.
49. Stolzmann P, Leschka S, Scheffel H, Krauss T, Desbiolles L, Plass A, et al. Dual-source CT in step-and-shoot mode: noninvasive coronary angiography with low radiation dose. Radiology. 2008. 249:71–80.
50. Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG. Technical principles of dual source CT. Eur J Radiol. 2008. 68:362–368.
51. Lell MM, May M, Deak P, Alibek S, Kuefner M, Kuettner A, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011. 46:116–123.
52. Geyer LL, Scherr M, Körner M, Wirth S, Deak P, Reiser MF, et al. Imaging of acute pulmonary embolism using a dual energy CT system with rapid kVp switching: initial results. Eur J Radiol. 2011. [Epub ahead of print].
53. Boll DT, Merkle EM, Paulson EK, Mirza RA, Fleiter TR. Calcified vascular plaque specimens: assessment with cardiac dual-energy multidetector CT in anthropomorphically moving heart phantom. Radiology. 2008. 249:119–126.
54. Chae EJ, Seo JB, Goo HW, Kim N, Song KS, Lee SD, et al. Xenon ventilation CT with a dual-energy technique of dual-source CT: initial experience. Radiology. 2008. 248:615–624.
55. Goo HW, Yang DH, Hong SJ, Yu J, Kim BJ, Seo JB, et al. Xenon ventilation CT using dual-source and dual-energy technique in children with bronchiolitis obliterans: correlation of xenon and CT density values with pulmonary function test results. Pediatr Radiol. 2010. 40:1490–1497.
56. Goo HW, Yang DH, Kim N, Park SI, Kim DK, Kim EA. Collateral ventilation to congenital hyperlucent lung lesions assessed on xenon-enhanced dynamic dual-energy CT: an initial experience. Korean J Radiol. 2011. 12:25–33.
57. Fink C, Johnson TR, Michaely HJ, Morhard D, Becker C, Reiser M, et al. Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo. 2008. 180:879–883.
58. Goo HW. Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr Radiol. 2010. 40:1536–1544.
59. Fletcher JG, Takahashi N, Hartman R, Guimaraes L, Huprich JE, Hough DM, et al. Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol Clin North Am. 2009. 47:41–57.
60. Primak AN, Giraldo JC, Eusemann CD, Schmidt B, Kantor B, Fletcher JG, et al. Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in vivo. AJR Am J Roentgenol. 2010. 195:1164–1174.
61. Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology. 2008. 249:671–681.
62. Kalra MK, Wittram C, Maher MM, Sharma A, Avinash GB, Karau K, et al. Can noise reduction filters improve low-radiation-dose chest CT images? Pilot study. Radiology. 2003. 228:257–264.
63. Mieville FA, Gudinchet F, Rizzo E, Ou P, Brunelle F, Bochud FO, et al. Paediatric cardiac CT examinations: impact of the iterative reconstruction method ASIR on image quality--preliminary findings. Pediatr Radiol. 2011. 41:1154–1164.
64. Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011. 21:2521–2526.
65. Kalra MK, Dang P, Singh S, Saini S, Shepard JA. In-plane shielding for CT: effect of off-centering, automatic exposure control and shield-to-surface distance. Korean J Radiol. 2009. 10:156–163.
66. Coursey C, Frush DP, Yoshizumi T, Toncheva G, Nguyen G, Greenberg SB. Pediatric chest MDCT using tube current modulation: effect on radiation dose with breast shielding. AJR Am J Roentgenol. 2008. 190:W54–W61.
67. Duan X, Wang J, Christner JA, Leng S, Grant KL, McCollough CH. Dose reduction to anterior surfaces with organ-based tube-current modulation: evaluation of performance in a phantom study. AJR Am J Roentgenol. 2011. 197:689–695.
68. Watanabe H, Kanematsu M, Miyoshi T, Goshima S, Kondo H, Moriyama N, et al. Improvement of image quality of low radiation dose abdominal CT by increasing contrast enhancement. AJR Am J Roentgenol. 2010. 195:986–992.
69. Deak PD, Smal Y, Kalender WA. Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology. 2010. 257:158–166.
70. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. 2006. Washington, DC: National Academies Press.
71. European Commission. European guidelines for quality criteria for computed tomography. EUR 16262 EN. 2000. Luxembourg: European Commission.
72. Shrimpton PC. Assessment of patient dose in CT: appendix C-European guidelines for multislice computed tomography. European Commission project MSCT: CT safety & efficacy-a broad perspective. 2004. Accessed on November 3, 2011. Available at: http://www.msct.ed/PDF_FILES/Appendix%20paediatric%20CT%20Dosimetry.pdf.
73. Tsai HY, Tung CJ, Yu CC, Tyan YS. Survey of computed tomography scanners in Taiwan: dose descriptors, dose guidance levels, and effective doses. Med Phys. 2007. 34:1234–1243.
74. American College of Radiology (ACR) practice guidelines for diagnostic reference levels in medical X-ray imaging. 2008. Accessed on November 3, 2011. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/RadSafety/RadiationSafety/guideline-diagnostic-reference.aspx.
75. Korea Institute for Accreditation of Medical Image. National survey of radiation dose of computed tomography in Korea. 2009.
76. Bauhs JA, Vrieze TJ, Primak AN, Bruesewitz MR, McCollough CH. CT dosimetry: comparison of measurement techniques and devices. Radiographics. 2008. 28:245–253.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download