Abstract
Objective
We wanted to evaluate the feasibility of catheter-directed thrombolysis with a continuous infusion of low-dose urokinase for treating non-acute (less than 14 days) deep venous thrombosis of the lower extremity.
Materials and Methods
The clinical data of 110 patients who were treated by catheter-directed thrombolysis with a continuous infusion of low-dose urokinase for lower extremity deep venous thrombosis was analysed. Adjunctive angioplasty or/and stenting was performed for the residual stenosis. Venous recanalization was graded by pre- and post-treatment venography. Follow-up was performed by clinical evaluation and Doppler ultrasound.
Results
A total of 112 limbs with deep venous thrombosis with a mean symptom duration of 22.7 days (range: 15-38 days) were treated with a urokinase infusion (mean: 3.5 million IU) for a mean of 196 hours. After thrombolysis, stent placement was performed in 25 iliac vein lesions and percutaneous angioplasty (PTA) alone was done in five iliac veins. Clinically significant recanalization was achieved in 81% (90 of 112) of the treated limbs; complete recanalization was achieved in 28% (31 of 112) and partial recanalization was achieved in 53% (59 of 112). Minor bleeding occurred in 14 (13%) patients, but none of the patients suffered from major bleeding or symptomatic pulmonary embolism. During follow-up (mean: 15.2 months, range: 3-24 months), the veins were patent in 74 (67%) limbs. Thirty seven limbs (32%) showed progression of the stenosis with luminal narrowing more than 50%, including three with rethrombosis, while one revealed an asymptomatic iliac vein occlusion; 25 limbs (22%) developed mild post-thrombotic syndrome, and none had severe post-thrombotic syndrome. Valvular reflux occurred in 24 (21%) limbs.
Treatment of non-acute (a symptom duration greater than 14 days) deep vein thrombosis (DVT) has long been a clinical problem. Because the thrombus has a tendency to adhere and organize after the acute stage, it is more difficult to dissolve and it often requires a longer time to be penetrated, and systemic thrombolysis is generally disappointing.
Catheter-directed thrombolysis (CDT) has the potential to improve the long-term outcomes in patients with DVT (1, 2). But the current CDT therapy is characterized by an initial bolus or pulse-spray injection of a high-dose thrombolytic agent followed by continuous infusion during a short time (3), and this type of therapy is suggested for treating acute DVT (≤ 14 days) (4). This therapy also has a potentially higher incidence of major bleeding (8%) (5). As a result, treatment of non-acute DVT can cause a woeful predicament.
In this study, we aimed at exploring a safe and effective therapy for non-acute DVT. We utilized CDT via the popliteal vein with a continuous infusion of low-dose urokinase for a longer time to treat selected non-acute lower extremity DVT, and this was combined with percutaneous angioplasty (PTA) and stent placement for an underlying venous stenosis or occlusion.
Between December 2002 and February 2008, the consecutive patients who had a first episode of proximal DVT and who underwent CDT were included for this prospective study. The diagnosis of DVT was confirmed by the characteristic clinical manifestations and the Doppler ultrasound and/or venographic findings. The patients who were eligible for enrollment had documented symptoms of lower extremity swelling and/or pain, a duration of symptoms beyond 14 days and they were positive for D-dimer. The exclusion criteria were isolated infrapopliteal DVT, recurrent ipsilateral DVT, pre-existing leg ulcers or signs of chronic venous insufficiency, a short life expectancy and contraindications to the use of contrast media, anticoagulation or thrombolytic agents. The contraindications to thrombolytic agents included active internal bleeding, recent cerebrovascular accident, recent serious trauma, recent major surgery (less than 2 weeks), allergy to thrombolytic agents, recent gastrointestinal bleeding, uncontrolled hypertension, pregnancy, bacterial endocarditis and coagulopathy. The study protocol was approved by the local ethics committee (China).
With the patient in the prone position, venous access was obtained through the ipsilateral popliteal vein under ultrasound guidance. A 5-Fr vascular sheath (Terumo, Japan) was placed and direct venography was performed to estimate the severity and extent of the DVT under fluoroscopy. A 5-Fr multiple-side-hole lysis catheter (AngioDynamics, Queensbury, NY) was then gently inserted with the tip embedded in the distal extent of the thrombus, and the catheter and sheath were fixed and joined with a microinjection pump. Urokinase (Tianjin Biochem Pharma Co., LTD, China) was continuously pumped in at a low dose of 50,000-100,000 IU/8 hours (it was constituted in 50 ml of 0.9% saline) through the catheter with the same dose of urokinase being simultaneously pumped through the sheath. Surveillance venography was performed every 2-3 days to access the degree of thrombolysis and to adjust the catheter position to facilitate effective thrombolysis. The fibrinogen level was monitored at varying intervals, and if the fibrinogen level dropped below 100 mg/dL, then further urokinase infusion was postponed to allow the fibrinogen to recover, and then the urokinase infusion was restarted at 50% of the original dose. After thrombolysis, further adjunctive procedures were done if there was an underlying iliac vein stenosis of greater than 50%. The adjunctive procedures consisted of PTA and stent placement. Before catheterization, we recommended prophylactic placement of a retrievable inferior vena cava (IVC) filter under local anesthesia through the right jugular vein or the contralateral femoral vein, and especially when patients had symptomatic pulmonary embolism, or the thrombus was free-floating or it had propagated into the IVC. If there were large amounts of thrombus in the iliac vein before PTA, then placing an IVC filter was recommended (6). In general, the IVC filter was removed within two weeks after the placement.
In the hospital, low molecular weight heparin (LMWH; Fraxiparine, Glaxo Smith Kline, Suzhou, China) was administered subcutaneously at the rate of 0.4-0.6 ml for 12 hours initially before CDT. Warfarin sodium (QILU Pharma Co., Ji'nan, China) was routinely started prior to hospital discharge and this was continued for at least six months, and the dose was adjusted to maintain an international normalized ratio (INR) of 2.0 to 3.0. The LMWH was discontinued when the INR reached the target value for two consecutive days and the patients had received it for at least five days. After hospital discharge, all the patients were recommended to wear gradual compression stockings (below-knee, 30-40 mmHg, Sigvaris Inc., Peachtree City, GA) for more than one year.
During the treatment, we recorded the affected limbs' circumference and the peri-procedure complications. We measured the thigh circumference at 15 cm above the knee joint and that of the calf at 10 cm below the tibial tuberosity. Complications were classified by the outcome with using the SIR (Society of Interventional Radiology) classification system for complications (7, 8).
The venograms were retrospectively reviewed by a consultant physician who was 'blinded' to the study's purpose. Venous recanalization was determined by comparing the thrombus scores (9) of the pre- and post-treatment venography, and this was categorized as follows: "complete" recanalization for 95-100% restoration of patency, "partial" for 50-95% and "minimal" for less than 50% due to residual stenosis or organized thrombus (10, 11). The recanalization was calculated after completion of treatment, which included the CDT and any additional adjunctive procedures.
Technical success was defined as restoration of patency and flow with less than 50% residual stenosis, and clinical success was defined as significant resolution of lower extremity pain and swelling. Follow-up visits were scheduled at three and six months, and every six months thereafter. At each visit, a patient underwent a clinical evaluation according to a modified Villalta scale (12), and a Doppler ultrasound assessment of the affected venous patency and reflux by a designated experienced vascular ultrasound physician. The presence of five leg symptoms (pain, cramps, heaviness, pruritus and paresthesia) and six objective signs (pretibial edema, skin induration, hyperpigmentation, new venous ectasia, redness and pain during calf compression) was scored. Each of the signs and symptoms were rated as 0 (absent), 1 (mild), 2 (moderate) or 3 (severe). Clinical evaluation outcomes were classified as follows: a total score greater than 14 points or a venous ulcer was defined as severe post-thrombotic syndrome (PTS); 5-14 points was given for mild PTS and less than five points was given for no PTS. Valvular reflux is defined as greater than 0.5 seconds for the valve closure time after distal compression and release using an ultrasonic probe in a non-weight-bearing limb with the patient in the standing position (13). Primary patency was defined as confirmed patency and < 50% restenosis as documented by Doppler ultrasound (14).
Of the 142 eligible patients with an episode of non-acute proximal DVT of the lower extremity, 32 were excluded because of previous ipsilateral DVT (n = 8), pre-existing leg ulcers or venous insufficiency (n = 13), a poor life expectancy (n = 5) or the inability to attend follow-up visits (n = 6). The remaining 110 patients were enrolled this study. The baseline characteristics are reported in Table 1. Written informed consent was obtained from each enrolled patient or the family after the purpose and risk of the treatment were fully explained.
As determined at venography, iliofemoral DVT was present in 94 (84%) of 112 limbs and among these four (4%) limbs had thrombus propagating into the IVC, and femoropopliteal DVT occurred in 18 (16%) limbs. Eighty-two patients received retrieval IVC filter placement (32 OptEase Filters, Cordis, Miami, FL; 50 Aegis Filters, Seercare, Shenzhen, China). After lysis, 63 filters were retrieved (no evidence of clot was observed within the filters) and the other 19 were implanted permanently (filling defects were observed within six filters and the other 13 were abandoned and not retrieving due to economic considerations). Urokinase infusion was postponed and the dosage was adjusted in seven patients due to the fibrinogen progressively dropping below 100 mg/dL. The mean duration of CDT was 196 hours (range: 96-272 hours) and the average total dose of urokinase was 3.5 million IU (range: 1.8-5.7 million IU).
Percutaneous angioplasty with stent placement (Wallstent, Boston Scientific Co., Natick, MA) was necessary to treat residual stenoses and/or short occlusions in the iliac segments that were resistant to CDT in 25 (22%) limbs, and another five received iliac vein PTA alone for economic reasons. After completion of treatment, complete recanalization was achieved in 31 (28%) infusions and partial recanalization was achieved in 59 (53%), while minimal recanalization was achieved in 22 (19%). Thus, 90 (81%) treated limbs achieved significant recanalization (≥ 50% recanalization). In addition, venography indicated well-developed venous collaterals around the partially recanalized veins. Figures 1, 2, 3, 4 illustrate the representative cases.
At discharge from hospital, considerable improvement of the swelling and pain of the affected limb was achieved in 96% (108 of 112) of the limbs. The thigh circumference of the affected limb decreased by 4.0 ± 1.6 cm, while the calf circumference decreased by 3.6 ± 1.8 cm (Table 2); the rate of detumescence was up to 75%. The mean hospital stay was 11.3 days (range: 7-15 days). The mean hospital expense was $9,000 (range: $5,300-11,000).
Overall, there was an immediate technical success rate of 81% (90 of 112) and a clinical success rate of 96% for the treated limbs. The treatment outcomes are listed in Table 3.
During the hospital stay, 14 (13%) patients experienced minor bleeding, of which bleeding at the puncture site occurred in 10 (9%) patients, and four (4%) patients had mucosal bleeding (2 cases of hematuria, 1 vaginal bleeding and 1 nose bleeding). Forty-one (37%) patients complained of slight pain in the affected knees (intramuscular hemorrhage or hemarthrosis was excluded by ultrasound and X-ray examination). None suffered from intracranial hemorrhage, symptomatic pulmonary embolism or other procedure-related complications.
The average duration of follow-up was 15.2 months (range: 3-24 months). During the period, the vein was patent in 74 (67%) limbs, including 23 with stent placement. The overall stent patency was 92% (23 of 25); 37 (32%) limbs showed progression of the stenosis with luminal narrowing more than 50%, including three limbs with rethrombosis, four with PTA alone and two with stent placement, while asymptomatic iliac vein occlusion occurred in one limb with PTA alone. Twenty five (22%) limbs developed mild PTS that mainly presented as heaviness and edema of the affected limbs after activities, and mild pruritus or hyperpigmentation was also present in five limbs, but none of these five limbs had severe PTS. The median total PTS score was three (range: 0-9) and 52 limbs (47%) had a score of 0. Valvular reflux occurred in 24 (21%) limbs (Table 4).
Systemic thrombolysis requires the thrombolytic agents to reach the position of the thrombus with blood flow through open veins to bring the thrombolytic agents into the play, and the thrombolytic effect declines greatly when the venous outflow is obstructed (15). CDT involves a focused delivery of thrombolytic agents directly through the side holes of a catheter traversing the thrombus and this may be more effective for local thrombolysis and restoration of patency (16). But for a thrombus tending to adhere and organize after the acute stage, the current CDT with high-dose thrombolytic agent administration in a short time (e.g., a mean of 7.8 million IU urokinase was administrated over an average of 51 hours at 100,000-370,000 IU/hour [3]) is usually not sufficient for non-acute DVT, and such administration has the potential for a higher bleeding rate.
This study used CDT with a continuous infusion of low-dose urokinase to treat non-acute lower extremity DVT. The mean infusion time (196 hours) was longer than that of a previously reported data (mean: 51 hours, range: 30-192 hours) (3), and the average urokinase dose was 3.5 million IU as compared to the average dose of 7.8 million IU reported in Mewissen's report (9). By the end of treatment, 81% of the infusions achieved significant recanalization of ≥ 50%, of which 28% achieved complete recanalization. During the mean 15.2 months of follow-up, the veins were patent in 74 (67%) limbs. Mewissen reported 36 (68%) infusions via CDT achieved significant recanalization in 53 limbs with "chronic" DVT with a duration of symptoms more than 10 days, of which 19% of the limbs achieved complete recanalization; for all the infusions, the primary patency rate was 60% at one year (9). In general, an acute thrombotic event in a patient without a history of DVT will most likely yield a favorable grade of lysis, which in turn will be predictive of continued patency. CDT should only be proposed when DVT has occurred less than 10 days from the time lytic therapy is available (9), but our results showed that CDT with continuous infusion of low-dose urokinase could also be applied to non-acute DVT. Thus, the inclusion window for the symptom duration in our study (15-38 days) was wider than that in the previous studies (≤ 14 days) (17, 18).
Because CDT alone does not always succeed in completely restoring venous patency, performing adjunctive procedures is common. These additional methods further diminish the clot burden and improve venous flow. This study combined CDT with PTA or/and stent placement for residual stenosis. The results showed that the effect of PTA alone was not desirable. After PTA, the stenotic vein often soon recoiled and stent placement could generally prevent that. During follow-up, the overall stent patency was 92% (23 of 25); however, all the five cases that received PTA alone developed restenosis or occlusion in the iliac veins, and this result was in accordance with that of the previous studies (19-21). So we also suggest stent placement in the iliac vein rather than angioplasty only.
In this study, most patients reported symptom relief with decreased swelling and pain and improvement of the limb status: the detumescence rate was up to 75%. But it deserves noting that in some patients, the degree of thrombolysis was not satisfactory whereas the clinical symptoms were obviously alleviated, which might be related to re-opening of the intravenous collaterals.
Urokinase is a kind of plasminogen activator that is given at the time of thrombolysis, and this drug can cause accentuation of the fibrinolytic system with a potential hemorrhagic risk. A higher incidence of bleeding is associated with using high-dose urokinase (22, 23). Mewissen et al. (9) reported on 473 DVT patients who were treated with catheter-directed urokinase (mean dose: 7.8 million IU) for a mean of 53.4 hours and they achieved favorable venous patency, yet bleeding complications were reported in 27% (131 of 473) of the patients and 11% (54 of 473) experienced major bleeding (9) that might have been related to a large dose of urokinase infused in a short time. In our study, CDT was characterized by a lower dose infusion for a longer time, and the venous patency we achieved was similar to that in Mewissen's report, but the incidence of minor bleeding was only 13% (14 of 112) without any major hemorrhages. The bleeding complication rate was also obviously lower than the 30% in a review of the previous pooled data (24). In addition, 41 (37%) patients complained of slight pain in the affected knees during CDT (intramuscular hemorrhage or hemarthrosis was excluded by ultrasound and X-ray examination), but the pain disappeared after the patients performed moderate exercise after removal of the catheters and the sheaths, and so this pain might have been related to limited mobility of the knee-joint.
When performing an endovenous operation, what needs to be stressed is that the operation should be done as gently as possible to reduce damage to the vein endothelium and valves and to avoid thrombus falling off due to the catheter or other apparatus. Placement of an IVC filter may prevent fatal pulmonary embolism due to venous return. However, some scholars think that filter placement is not associated with a reduced incidence of symptomatic pulmonary embolism (25). In this study, none of the patients suffered from symptomatic pulmonary embolism, including the 28 patients who didn't receive IVC filter placement. However, physicians should be aware that pulmonary embolism is a serious complication that can cause death, and so an endovascular operation must be normative and gentle. With the findings of free-floating thrombus in veins, we strongly recommend placement of an IVC filter prior to the operation.
Post-thrombotic syndrome is a chronic condition characterized by leg edema, pain, varicose veins, skin induration and venous leg ulcers (26). It develops in 20-50% of the patients within one to two years after the development of symptomatic DVT, of which five to 10% of these patients will develop severe PTS (27, 28). In our study, 25 (22%) patients suffered from mild PTS with the main manifestations of limb heaviness and edema after activities and none of them had severe PTS; valvular reflux occurred in 21% of the limbs in our study as compared to 37% (39 of 106) at one year in Markel's report (29). This reflects the ability of CDT to effectively preserve valve function and protect against the development of PTS. We presumed that the mechanism for this might be related to urokinase inhibiting the thrombus-induced inflammatory response through reducing the cytokines and adhesion molecules in the venous wall along with thrombus clearance and recanalizing veins, which in return maintained the functional integrity of the venous endothelium and smooth muscle cells (30). But it is suggested that careful long-term surveillance of the venous function is highly recommended after performing CDT.
In conclusion, our study results showed that CDT with a continuous infusion of low-dose urokinase was effective in removing the clot burden and restoring the venous flow for the patients with non-acute lower extremity DVT, and our method reduced the incidence of bleeding complications and resulted in desirable mid-term outcomes. To a certain degree, the therapy extended the time window of thrombolysis therapy, but the drawback was the longer hospital stay and the higher expense.
Figures and Tables
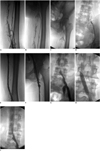 | Fig. 1Complete lysis in 33-year-old woman with 3-week history of swelling of left leg after cesarean section (venograms with patient in prone position).
A-D. Venograms obtained before catheter-directed thrombolysis show part or complete absence of contrast material in superficial femoral vein, common femoral vein, external iliac vein and common iliac vein. E-I. Venograms obtained after 240 hours of catheter-directed thrombolysis show deep veins (from superficial femoral vein to external iliac vein) with restored patency; (G) complete occlusion in proximal segment of common iliac vein; (H) common iliac vein received percutaneous angioplasty and (I) common iliac vein has restored patency after percutaneous angioplasty.
|
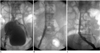 | Fig. 2Complete lysis in 26-year-old man with 16 days history of pain and swelling of both legs.
A-C. Venograms were obtained with patient in prone position, and these are the images shown. Venogram obtained before catheter-directed thrombolysis shows complete occlusion of dual iliac veins (A). Venograms obtained after 192 hours of catheter-directed thrombolysis show complete lysis (B) in right iliac vein and (C) in left iliac vein.
|
 | Fig. 3Iliac vein's restored patency after catheter-directed thrombolysis and percutaneous angioplasty with stenting in 53-year-old man who presented with edema of left lower extremity for 35 days.
A-C. Images were taken with patient prone on angiography table and these are images shown. Before catheter-directed thrombolysis, note severe left common iliac vein stenosis (arrow), complete absence of contrast material in external iliac vein (curved arrows) and thrombosis in inferior vena cava (open arrows) (A). After 196 hours of catheter-directed thrombolysis, thrombus in inferior vena cava was completely dissolved and external iliac vein obtained complete patency (B), and common iliac vein stenosis was treated with stent placement (arrow) and then common iliac vein achieved complete patency (C).
|
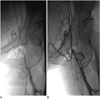 | Fig. 4Well-developed venous collaterals around recanalized femoral vein in 68-year-old man who presented with swelling of left leg for 30 days.
A. Before catheter-directed thrombolysis, common femoral vein was completely occluded and there was small number of venous collaterals around it. B. After 216 hours of catheter-directed thrombolysis, common femoral vein's patency was restored and large number of venous collaterals opened around it.
|
Table 3
Treatment Outcomes (112 Limbs in 110 patients)

Note.- CDT = catheter-directed thrombolysis, PTA = percutaneous angioplasty
*Complications were classified according to scoring system of Society of Interventional Radiology (SIR) (6, 7): Minor: A. No therapy, no consequence, B. Nominal therapy, no consequence, includes overnight admission for observation only; Major: C. Required therapy, minor hospitalization (< 48 hours), D. Required major therapy, unplanned increase in level of care, prolonged hospitalization (> 48 hours), E. Permanent adverse sequelae, and F. Death.
Acknowledgment
The authors would like to thank Drs. Shiyi Zhang, Zhenyue Zhong, Mo Wang and Hai Yuan, and Yan Sun MS in the Department of Vascular Surgery, Shandong Provincial Hospital, for their assistance with the data collection.
References
1. Blum A, Roche E. Endovascular management of acute deep vein thrombosis. Am J Med. 2005. 118:31S–36S.
2. Baekgaard N, Broholm R, Just S, Jørgensen M, Jensen LP. Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010. 39:112–117.
3. Grossman C, McPherson S. Safety and efficacy of catheter-directed thrombolysis for iliofemoral venous thrombosis. AJR Am J Roentgenol. 1999. 172:667–672.
4. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence Based Clinical Practice Guidelines (8th Edition). Chest. 2008. 133:454S–545S.
5. Vedantham S, Millward SF, Cardella JF, Hofmann LV, Razavi MK, Grassi CJ, et al. Society of Interventional Radiology position statement: treatment of acute iliofemoral deep vein thrombosis with use of adjunctive catheter-directed intrathrombus thrombolysis. J Vasc Interv Radiol. 2006. 17:613–616.
6. Lou WS, Gu JP, He X, Chen L, Su HB, Chen GP, et al. Endovascular treatment for iliac vein compression syndrome: a comparison between the presence and absence of secondary thrombosis. Korean J Radiol. 2009. 10:135–143.
7. Patel N, Sacks D, Patel RI, Moresco KP, Ouriel K, Gray R, et al. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Interv Radiol. 2003. 14:S453–S465.
8. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003. 14:S199–S202.
9. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999. 211:39–49.
10. Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006. 17:417–434.
11. Park YJ, Choi JY, Min SK, Lee T, Jung IM, Chung JK, et al. Restoration of patency in iliofemoral deep vein thrombosis with catheter-directed thrombolysis does not always prevent post-thrombotic damage. Eur J Vasc Endovasc Surg. 2008. 36:725–730.
12. Prandoni P, Lensing AW, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004. 141:249–256.
13. Mattos MA, Sumner DS. Gloviczki P, Yao JS, editors. Direct noninvasive tests (duplex scan) for the evaluation of chronic venous obstruction and valvular incompetence. Handbook of venous disorders. 2001. 2nd ed. New York, NY: Arnold;120–131.
14. Kölbel T, Lindh M, Akesson M, Wassèlius J, Gottsäter A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009. 16:483–491.
15. See-Tho K, Harris EJ Jr. Thrombosis with outflow obstruction delays thrombolysis and results in chronic wall thickening of rat veins. J Vasc Surg. 1998. 28:115–122.
16. Gogalniceanu P, Johnston CJ, Khalid U, Holt PJ, Hincliffe R, Loftus IM, et al. Indications for thrombolysis in deep venous thrombosis. Eur J Vasc Endovasc Surg. 2009. 38:192–198.
17. Beygui RE, Olcott C 4th, Dalman RL. Subclavian vein thrombosis: outcome analysis based on etiology and modality of treatment. Ann Vasc Surg. 1997. 11:247–255.
18. Kim HS, Patra A, Paxton BE, Khan J, Streiff MB. Catheter-directed thrombolysis with percutaneous rheolytic thrombectomy versus thrombolysis alone in upper and lower extremity deep vein thrombosis. Cardiovasc Intervent Radiol. 2006. 29:1003–1007.
19. Vedantham S, Vesely TM, Sicard GA, Brown D, Rubin B, Sanchez LA, et al. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2004. 15:565–574.
20. Jackson LS, Wang XJ, Dudrick SJ, Gersten GD. Catheter-directed thrombolysis and/or thrombectomy with selective endovascular stenting as alternatives to systemic anticoagulation for treatment of acute deep vein thrombosis. Am J Surg. 2005. 190:864–868.
21. Kwak HS, Han YM, Lee YS, Jin GY, Chung GH. Stents in common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005. 16:815–822.
22. Bjarnason H, Kruse JR, Asinger DA, Nazarian GK, Dietz CA Jr, Caldwell MD, et al. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997. 8:405–418.
23. Ouriel K, Gray B, Clair DG, Olin J. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000. 11:295–298.
24. Alesh I, Kayali F, Stein PD. Catheter-directed thrombolysis (intrathrombus injection) in treatment of deep venous thrombosis: a systematic review. Catheter Cardiovasc Interv. 2007. 70:143–148.
25. Segal JB, Streiff MB, Hofmann LV, Thornton K, Bass EB. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007. 146:211–222.
26. Kahn SR, Solymoss S, Lamping DL, Abenhaim L. Long-term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. J Gen Intern Med. 2000. 15:425–429.
27. Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004. 164:17–26.
28. Kahn SR. The post-thrombotic syndrome: the forgotten morbidity of deep venous thrombosis. J Thromb Thrombolysis. 2006. 21:41–48.
29. Markel A, Manzo RA, Bergelin RO, Strandness DE Jr. Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992. 15:377–382.
30. Cho JS, Martelli E, Mozes G, Miller VM, Gloviczki P. Effects of thrombolysis and venous thrombectomy on valvular competence, thrombogenicity, venous wall morphology, and function. J Vasc Surg. 1998. 28:787–799.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download