Abstract
Omental infarction occurring after open and laparoscopic-assisted distal gastrectomy with partial omentectomy for gastric cancer was a very rare disease in the past, but its incidence has increased as more partial omentectomies are now being performed. But there are few case reports or radiologic studies on its increasing incidence. It is necessary to differentiate omental infarction from carcinomatosis peritonei, since both have similar imaging findings. In this report, we describe two cases of omental infarction; each occurred after open and laparoscopic-assisted distal gastrectomy in early gastric cancer patients. Partial omentectomy was performed in both cases. Omental infarction following distal gastrectomy with partial omentectomy can be discriminated from carcinomatosis peritonei by comparing with different initial and follow up CT findings.
The conventional surgery for curative treatment of distal gastric cancer is resection of the distal stomach with all of the greater omentum and lesser omentum. But a less invasive procedure such as laparoscopic-assisted distal gastrectomy (LADG) has become more popular as the detection rate of early gastric cancer has increased as a result of widespread regular check-ups.
Laparoscopic surgery has become more popular as a minimal invasive procedure and it has been well demonstrated to have the advantages of a more favorable clinical course and lower mortality and morbidity, and especially for early gastric cancer patients (1, 2).
As for the techniques, LADG and conventional open gastrectomy are the same for the extent of gastrectomy and lymph node (LN) dissection. But unlike conventional open gastrectomy, the greater omentum is resected about 4-5 cm away from the greater curvature in LADG (2). The reasons are as follows: the possibility of omental metastasis is very low because the majority of patients who undergo LADG have early gastric cancer, and complete resection of the bulky greater omentum is technically difficult in the restricted space. Partial omentectomy is sometimes performed during open distal gastrectomy (ODG) because of the low potential for omental metastasis in patients with early gastric cancer and to save the operation time required for complete removal of the omentum.
As ODG and LADG with partial omentectomy are now being widely performed in Korea and Japan, it has increased the potential for omental infarction following partial omentectomy. Yet there are no radiologic studies or case reports of omental infarction following ODG and LADG with partial omentectomy for gastric cancer. So, we describe here the clinical features and computed tomography (CT) findings of omental infarction following partial omentectomy in open and laparoscopic-assisted distal gastrectomy and the differential diagnostic points from omental metastasis according to these two cases.
A 65-year-old woman underwent ODG with partial omentectomy and D1+ β lymph node dissection [Dissection of the group 1 nodes plus the left gastric artery, the anterior common hepatic artery and the celiac artery nodes. The grouping of LNs is according to the Japanese Classification for Gastric Carcinoma (JCGC) staging system by the primary location of cancer.] (3) for early gastric cancer localized in the mucosa (T1aN0M0). Her past medical history was diabetic mellitus and hypertension for several years that were controlled with medication and she underwent endoscopic submucosal resection (ESR) three times for gastric polyps. This patient was obese with a body mass index (BMI) 34.8 kg/m2 (an initial weight of 81.4 kg and a height of 153.2 cm). She did well without any complications after surgery.
Contrast enhanced abdominal CT and F18-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) were routinely performed to evaluate tumor recurrence at six months after surgery. On the contrast enhanced axial CT scan, an ill-defined area of haziness with hyperattenuating streaky infiltration was seen in the remnant great omentum at the left subphrenic space and lateral to the stomach (Fig. 1A). The patient had no any clinical symptoms and no abnormal lab findings. This CT finding was very similar to carcinomatosis peritonei. But it could be differentiated from carcinomatosis peritonei clinically because the lesion was locally confined to the remnant greater omentum and it was found in an early gastric cancer patient. In addition, the F18-FDG PET-CT scan showed no abnormal FDG uptake at the same lesion (Fig. 1B). So we decided to observe her without treatment and to conduct regular follow up. Six months after that, the follow up contrast enhanced CT scan revealed a decrease in the lesion size and there was progression from an ill-defined, heterogeneous fat density lesion to a well-defined, smaller lesion with a hyperdense rim at the left subphrenic space (Fig. 1C).
A 68-year-old woman underwent LADG with partial omentectomy and D1+ β lymph node dissection for early gastric cancer localized in the submucosa (T1bN1M0). Her past medical history was atrial fibrillation and Parkinson's disease for several years and these maladies were controlled with medication. This patient was thin with a BMI 19.7 kg/m2 (the initial weight of 50.4 kg and a height of 160 cm). On postoperative day 10, she developed localized right upper quadrant pain with leukocytosis of 17,090/mm3. She had no fever, chills or gastrointestinal symptoms such as nausea, vomiting or diarrhea other than the right upper quadrant pain.
Contrast enhanced abdominal CT was performed to differentiate the causes of her acute abdominal pain. The enhanced CT scan revealed a 4 cm sized heterogeneous fat density mass with a peripheral enhancing rim and streaky infiltration in the omentum at the anterior to the pancreas head, which was a reasonable finding of omental infarction (Fig. 2A). She improved after administration of anti-inflammatory agents and analgesics. After five days, her symptoms disappeared completely and she was discharged from the hospital.
After five months, she was followed up with CT and F18-FDG PET-CT. The previously noted lesions had almost resolved and there was no abnormal FDG uptake except only mild omental haziness (Fig. 2B, C).
Omental infarction is the end result of impaired perfusion to the greater omentum, and this is a rare disease entity that can cause acute or subacute abdominal pain in adults (4). Classified omental infarction into the primary and secondary types. The cause of primary idiopathic omental infarction is unknown, but it may be related to obesity, local trauma, occupational vibration, heavy food intake, excessive exercise and the use of laxatives. Obesity has especially been postulated as an important risk factor (5). Obesity causes irregularly distributed accumulations of excess omental fat and the increased fat deposits outstrip the blood supply to the thickened omentum. Secondary omental infarction can be induced by various causes: torsion due to adhesion between the omentum and pathologic foci such as surgical scars, inflammation, cysts and tumors, thrombosis due to hypercoagulopathy and vascular abnormalities and congestion of the mesenteric vein due to systemic diseases such as right-sided heart failure (6-8).
Omental infarction has been reported to occur more frequently on the right side, so clinically it is difficult to differentiate it from either appendicitis or cholecystitis. The reason may be that the omentum is longer and more mobile on the right side than on the left side (9). However, compared with the usual location and symptoms of the other omental infarctions, omental infarction after ODG or LADG with partial omentectomy is located in the mid-line or on the left side of the abdomen and it does not show clinical symptoms at diagnosis in the majority of patients such as our case 1. This is because omental infarction is affected by the volume and anatomical location of the remnant omentum rather than the anatomical location of the ligated vessel (10).
Omental infarction following ODG or LADG with partial omentectomy occurs at the remnant greater omentum. The residual area and the abundance of the blood supply for the remnant greater omentum are two important factors that affect the incidence of omental infarction. The blood supply of the greater omentum travels largely through the right and left gastroepiploic arteries. Omental infarction is caused by ligation of both the right and left gastroepiploic arteries. Of course, both gastroepiploic arteries were ligated in our two cases. Usually distal gastrectomy is performed in a patient with early gastric cancer that is localized in the distal stomach and there is very low possibility of omental metastasis. The left gastroepiploic LNs (4sb LN) are classified to the group 3 regional LNs for distal third primary gastric cancer according to the JCGC staging system, and the 4sb LN are not included in D2 dissection [Grouping of LNs is according to the Japanese Classification for Gastric Carcinoma (JCGC) staging system by the primary location of cancer.] for distal third primary gastric cancer (3). So the right gastroepiploic artery is ligated in almost all cases, but on the other hand, the left gastroepiploic artery can be preserved with subtotal gastrectomy. In addition and anatomically, the greater omentum has a extensive distribution of blood vessels that supply more than the basic metabolic requirements and it sometimes has collateral vessels from the middle colic artery or the superior mesenteric artery. Infarction does not usually occur even though most vessels are blocked. For these reasons, the incidence of secondary omental infarction following distal gastrectomy and partial omentectomy for gastric cancer, either open or laparoscopic assisted, is very low (10). But as laparoscopic surgery is now more commonly performed, the incidence of omental infarction following partial omentectomy with right and left gastroepiploic artery ligation has increased (7, 8).
The diagnosis of omental infarction is primarily based on the CT findings of an ill-defined heterogeneous mass or interspersed fatty lesion with hyperattenuating streaky infiltration located in the omentum in the early stage, and this progresses to a well-defined, smaller lesion with a hyperdense rim in the late phase (9). These findings can mimic carcinomatosis peritonei in the early phase and peritoneal epiploic appendagitis (PEA) in the late phase. Clinically, it is important to distinguish omental infarction and carcinomatosis peritonei, and especially for an incidentally detected lesion without any clinical symptoms at follow up CT in gastric cancer patient after distal gastrectomy. In this situation, making the differentiation from carcinomatosis peritonei is as follows: part of the omental infarction shows normal findings on the PET scan, the localized infarcted mass is confined to the remnant omentum, it is progressively smaller in size on the follow up CT without any treatment such as chemotherapy, omental infarction shows different imaging findings depending on the clinical phase, and it appears as ill-defined heterogeneous haziness that mimic carcinomatosis peritonei in the early phase, but it gradually turns into a well-defined fatty mass with a hyper-enhancing peripheral rim that mimics PEA, such as in our cases (9).
The pathologic diagnosis was not established in our two patients. Because the CT scans demonstrated the pathognomonic findings and a natural progressive course of this disease, the diagnosis of omental infarction was not difficult. In many cases, CT is helpful to make a confident diagnosis and so it allow conservative management, and a tissue diagnosis is uncommon. Further reports on pathologic confirmation are needed to evaluate in detail the disease pathophysiology of omental infarction after ODG and LADG.
In conclusion, omental infarction following ODG or LADG with partial omentectomy for gastric cancer is very rare, but the incidence is increasing with more widespread use of this procedure. The CT findings of omental infarction are similar to carcinomatosis peritonei in the early phase. Yet omental infarction following distal gastrectomy with partial omentectomy can be discriminated from carcinomatosis peritonei by the different clinical situation, the different CT findings, the different PET-CT findings and when comparing these findings with the follow up CT.
Figures and Tables
Fig. 1
Omental infarction after open distal gastrectomy.
A. Post-operative 6 months follow-up contrast enhanced abdominal CT scan shows typical area of ill-defined interspersed fatty lesion with hyperattenuating streaky infiltration in left subphrenic space which is remnant greater omentum (arrow). B. Post-operative 6 months follow-up axial F18-FDG PET-CT scan shows no abnormal FDG uptake at heterogeneous fatty lesion on contrast enhanced CT scan (arrow). C. Axial contrast enhanced CT performed 6 months later shows decrease in size and development of hyperdense rim surrounding omental infarct (arrows).
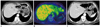
Fig. 2
Omental infarction after laparoscopic-assisted distal gastrectomy.
A. Contrast enhanced abdominal CT of patient with right upper abdominal pain at postoperative day 10. Axial contrast enhanced CT shows about 4 cm sized ill-defined heterogeneous fat mass with peripheral enhancing rim and streaky infiltration in omentum, anterior to pancreas head consistent with omental infarction (arrow). This mass closely abut on duodenal stump, but no abnormal wall thickening or inflammatory change in duodenal stump. B, C. Follow-up contrast enhanced CT (B) and PET-CT (C) at 6 months later show almost regression of previous noted lesions with remained mild omental streaky infiltration and no abnormal FDG uptake at remnant greater omentum (arrows).

References
1. Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001. 234:279–289. discussion 289-291.
2. Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007. 33:700–705.
3. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English Edition. Gastric Cancer. 1998. 1:10–24.
4. Puylaert JB. Right-sided segmental infarction of the omentum: clinical, US, and CT findings. Radiology. 1992. 185:169–172.
5. Theriot JA, Sayat J, Franco S, Buchino JJ. Childhood obesity: a risk factor for omental torsion. Pediatrics. 2003. 112:e460.
6. Wiesner W, Kaplan V, Bongartz G. Omental infarction associated with right-sided heart failure. Eur Radiol. 2000. 10:1130–1132.
7. Bestman TJ, Valk JW, Gypen B, Declercq S, Hendrickx L. An unusual complication after Roux-en-Y gastric bypass: torsion and infarction of the divided Omentum. Obes Surg. 2009. 19:1731–1733.
8. Dallal RM, Bailey LA. Omental infarction: a cause of acute abdominal pain after antecolic gastric bypass. Surg Obes Relat Dis. 2006. 2:451–454.
9. Singh AK, Gervais DA, Lee P, Westra S, Hahn PF, Novelline RA, et al. Omental infarct: CT imaging features. Abdom Imaging. 2006. 31:549–554.
10. Kim MC, Jung GJ, Oh JY. Omental infarction following laparoscopy-assisted gastrectomy (LAC) for gastric cancer. J Korean Gastric Cancer Assoc. 2010. 10:13–18.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download