Abstract
Bilateral carotid and vertebral rete mirabile (CVRM) is a very rare condition. We report a new case of CVRM initially detected by magnetic resonance imaging (MRI) of the cervical spine. MRI demonstrated tortuous vascular signal voids limited to the anterior cerebrospinal fluid space mimicking spinal dural arteriovenous fistula. A diagnosis of CVRM was confirmed on the basis of angiographic findings of rete formation associated with bilateral aplasia of the cavernous internal carotid and vertebral arteries without abnormal arteriovenous connection.
Rete mirabile (RM) is an arterial or arteriolar meshwork formed by branches from one or more arteries that converge into a single artery. Carotid rete mirabile (CRM) is a physiological network between the external and internal carotid systems that are usually located around the cavernous sinus in lower mammals, such as goats, sheep, and pigs (1). CRM is a very rare pathological condition in humans and is associated with aplasia or hypoplasia of the internal carotid artery (ICA), and carotid and vertebrobasilar RM (CVBRM) is even rarer. To the best of our knowledge, only nine cases, including six of vertebral rete (VR) and three of basilar rete (BR), have been reported. Some of the patients with CVBRM presented with subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or ischemia, but others were asymptomatic. When asymptomatic, the conditions were detected only incidentally on computed tomography (CT) or magnetic resonance image (MRI) of the brain. We report the case of a patient with carotid and vertebral RM (CVRM) that was initially detected on MRI of the cervical spine.
A 28-year-old man presented with non-specific posterior neck pain radiating to the shoulder and arm, but not accompanied by sensory or motor changes. The patient was previously healthy with an unremarkable past medical history and no history of trauma. A neurological examination revealed no abnormal features. Non-contrast cervical MRI demonstrated tortuous vascular signal voids limited to the anterior cerebrospinal fluid (CSF) space on T2-weighted images (Fig. 1A). Neither abnormal intramedullary signal intensity, nor spinal cord swelling was observed by MRI. The CT angiography (CTA) performed to evaluate for abnormal vasculature showed a dilated and tortuous anterior spinal artery, focal interruption, and fusiform dilatation at the V4 segment of the vertebral artery (Fig. 1B). CTA demonstrated hypoplastic cervical ICA on the right side and relatively normal cervical ICA on the left side. The carotid canals were asymmetrically small on the right side (Fig. 1C). A catheter angiography was performed to investigate a spinal arteriovenous fistula (AVF) or arteriovenous malformation (AVM). Angiography of the right common carotid artery (RCCA) demonstrated long segmental narrowing of the right ICA (RICA) from the proximal cervical portion and occlusion at the cavernous segment with trans-dural rete formation through the external carotid artery (ECA) branches (Fig. 1D, E). Selective angiography of the RICA was not attempted due to narrowing of the proximal ICA. Angiography of the left ICA (LICA) demonstrated segmental absence of the cavernous ICA with surrounding rete formation, while all segments of the LICA except for the cavernous portion, middle cerebral artery (MCA), and anterior cerebral artery (ACA) showed normal features. The distal basilar and posterior cerebral arteries are being filled by the posterior communicating artery and the persistent trigeminal artery (Fig. 1F). A left ECA angiography demonstrated transdural rete formation between the internal maxillary artery and the enlarged ophthalmic artery (Fig. 1G, H). A right vertebral artery (RVA) angiography showed segmental absence at the V3 segment of the RVA with surrounding tortuous dilated rete formation. A saccular aneurysm at the distal portion of the rete and fusiform dilatation of the V4 segment of the RVA was demonstrated. The distal RICA and right MCA supplied by the posterior communicating artery (P-com) on RVA angiography had a normal configuration (Fig. 1I, J). The radiculomedullary artery (RMA) originating at the proximal RVA (at the level of the seventh cervical vertebra) was enlarged and connected via a prominent anterior spinal artery (Fig. 1K). A left vertebral artery (LVA) angiography revealed similar findings to those of the RVA angiography, with less prominent rete formation at the V3 segment level and RMA origination at the proximal LVA (at the level of the fifth cervical vertebra) (Fig. 1L). Other selective angiographies never showed abnormal arteriovenous connections suggestive of AVF or AVM. Bypass surgery was not attempted because symptoms were mild and the brain perfusion status was relatively normal.
Agenesis, aplasia, and hypoplasia of the ICA are rare. Agenesis is defined as the complete failure of development of an organ; aplasia, lack of development; and hypoplasia, incomplete development (2). Segments of the fully developed ICA correspond to embryonic structures. In segmental agenesis of the ICA, each of these embryonic arteries is a potential source of reconstitution of distal flow in the ICA (3). In the pathogenesis of CRM formation, the most accepted theory is the late (fetal or perinatal) regression of the artery (2, 3). Mahadevan et al. (3) postulated that if agenesis is the primary deficit, the embryonic vessels should be the primary source of recruitment for collateral circulation. If the absence of the ICA occur by regression after its development, the embryonic vessels are already regressed and no longer available for collateral circulation. As the CRM is not present in any human developmental state (3-6), it is probably a secondary change to ICA regression in early development (6). Evaluation of the skull base for presence or absence of the carotid canal is required for distinguishing aplasia from agenesis, since the presence of the ICA precursor is a prerequisite for the development of the carotid canal at 5-6 weeks of gestation. Demonstration of a small carotid canal by CT is helpful for excluding acquired etiologies; the thin and hypoplastic carotid canal demonstrated on CT supports this theory (6). In our case, both carotid canals were demonstrated on CT even though it was very small on the right side and relatively normal on the left side. This CT finding supports the theory that the pathogenesis of CRM is not agenesis, but the later regression of the artery.
In this case, we could rule out the pseudorete by acquired atherosclerotic occlusion for several reasons; 1) the level of segmental agenesis is bilaterally symmetrical; 2) all cervical and cerebral arteries except hypoplastic right cervical ICA showed normal features without stenosis or luminal irregularity; 3) the patient was very young and had no risk factors for atherosclerosis such as diabetes mellitus, hypertension, hyperlipidemia, and smoking.
Sahin et al. (2) described six pathways of collateral circulation in association with ICA absence. Type F refers to collateral flow to the distal ICA via transcranial anastomoses from the maxillary branches of the ECA as well as tiny ICA collaterals, which are called rete mirabile (in Latin, "wonderful net").
Carotid rete mirabile has the following characteristic features on angiogram: (i) hypoplastic ICA, (ii) arterial plexus between the maxillary artery and cavernous portion of the ICA, (iii) dilated ophthalmic artery, (iv) supraclinoid ICA that is not hypoplastic and is fed by the arterial plexus and ophthalmic artery, (v) bilateral lesions, and (vi) no abnormal vessels such as moyamoya in the intradural circulation (2, 7). In our case, all of these features were identified on angiography.
Agenesis of the vertebral artery with associated rete compensation is even rarer than ICA agenesis. Most VRM occurs at the transdural segment with collateral circulation from the muscular and meningeal branches. To our knowledge, there are nine cases in the literature of carotid and vertebrobasilar rete mirabile in the literature (Table 1). Transdural rete formation was associated with vertebral agenesis in six cases (3, 4, 7-10), while intradural rete formation associated with basilar agenesis occurred in two cases (2, 6), and vertebrobasilar agenesis occurred in one (5). In only two of these cases, angiography demonstrated both transdural anastomosis via dilated anterior spinal or medullary arteries and intradural rete formation (3, 7).
In our case, MRI of the cervical spine was performed as an initial examination, and we suspected spinal dural AVF. However, unlike typical AVF, MRI showed multiple vascular signal voids in only the anterior aspect of the spinal cord, and the dilated vessels were larger in our case than in spinal AVF. CRM can appear as moyamoya disease on brain MRI because it demonstrates dot-like signal voids at the basal cistern; however, the important difference between CRM and moyamoya disease is that MRI reveals normal supraclinoid ICA, MCA, and ACA signal voids in CRM.
Some mammals, such as cats, sheep, goats, oxen, and pigs, have well-developed CRM as a part of their normal physiology (to prevent overheating of the brain and regulate the pressure and flow of the cerebral blood circulation). In humans, however, CVBRM can be a clinically significant condition because it frequently presents with hemorrhagic and ischemic manifestations at a relatively young age. According to Li et al. (7), 27 patients with CRM had variable presentations: 12 with ischemic stroke, 11 with SAH, and four with intracerebral hemorrhage (ICH). Of the nine patients with CVBRM, three presented with ischemic events including ischemic stroke in two (a 40-year-old man and 34-year-old woman) and temporary blindness in one (a 29-year-old woman). In addition, one patient presented with carotid-cavernous fistula (a 20-year-old man), one patient presented with subarachnoid hemorrhage (a 37-year-old woman), and one patient presented with intracerebral hemorrhage (a 57-year-old woman). The remaining three patients were asymptomatic, and CVBRM was incidentally detected in a brain CT performed after a traffic accident, or in an angiography performed for tumor embolization, or during diagnosis of AVM. Our patient presented with nonspecific posterior neck pain with no accompanying neurological symptoms or signs. His neck pain may bear no relation with CVBRM. Associated lesions in patients with CRM include intracranial aneurysm, AVM, carotid-cavernous fistula, Dieulafoy's ulcer, pseudoxanthoma elasticum, and posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta, and cardiac defects and eye abnormalities (PHACE) syndrome. However, only two patients with CVBRM had associated lesions including AVM and pseudoxanthoma elasticum (8, 9). To the best of our knowledge, this is the first case where an aneurysm was demonstrated on angiography even though some cases presenting with SAH or ICH have been reported. Rupture of an aneurysm or anastomosing vessel and hemodynamic stress may cause SAH; however, endovascular or surgical treatment is almost impossible because of tortuous and fragile collateral vessels (2).
We must recognize that CVBRM, although rare, is an important cause of strokes in young adults. An ischemic or hemorrhagic stroke is a major presentation, and can be accompanied by non-specific symptoms such as dizziness or blindness. We could also incidentally encounter this condition on MRI-MRA or CT-CTA performed for another reason. As a result, we need to be aware of the specific MRI or CT imaging and angiographic findings for this condition.
Figures and Tables
Fig. 1
Bilateral carotid and vertebral rete mirabile in 28-year-old man.
A. T2-weighted images of cervical spine MRI showing tortuous and dilated vascular signal voids (arrows) limited to anterior portion of spinal cord. B. Sagittal reconstructed maximum-intensity-projection image of CT angiography demonstrating dilated anterior spinal artery in spinal canal (black arrow) and fusiform dilatation of right distal vertebral artery (white arrow). C. Coronal reconstructed maximum-intensity-projection image and volume rendering image of CT angiography show hypoplastic cervical internal carotid artery and small carotid canal on right side (white open arrows) compared with normal cervical internal carotid artery and normal carotid canal on left side (black open arrows). D, E. Right common carotid artery angiography demonstrates diffuse narrowing of right internal carotid artery (thin arrows) and occlusion at level of cavernous internal carotid artery (thick arrow) accompanying transdural rete formation (open arrow) through external carotid artery branches. F. Left ICA angiography demonstrates segmental absence of cavernous ICA (white long arrow) with surrounding rete formation (open arrows). Distal basilar and posterior cerebral arteries (white short arrows) are being filled by posterior communicating artery (double arrowheads) and persistent trigeminal artery (arrowhead). All segments of left ICA except for cavernous segment and cerebral arteries appear normal. G, H. Left external carotid artery angiography demonstrates transdural rete formation (arrows) between internal maxillary artery and enlarged ophthalmic artery (arrowhead). I-L. Right (I, K, AP views; J, lateral view) and left vertebral artery angiography (L, AP view) show segmental agenesis at V3 segment (white arrow) with surrounding rete formation (black, open arrow). Saccular aneurysm (black arrowhead) at distal portion of rete and fusiform dilatation of V4 segment (black arrow) are demonstrated on right vertebral artery angiography. Enlarged radiculomedullary arteries (white arrowheads) are connected by dilated anterior spinal artery (double arrowheads) that is developed as collateral to asplastic segment. Right distal internal carotid and middle cerebral arteries supplied by posterior communicating artery (white, open arrows), appear normal.
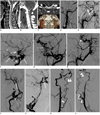
Table 1
Reported Cases of Bilateral Carotid and Vertebrobasilar Rete Mirabile

Note.- AVM = arteriovenous malformation, BA = basilar artery, CCF = carotid cavernous fistula, CTA = CT angiography, ICA = internal carotid artery, ICH = intracerebral hemorrhage, MRA = MR angiography, Ref. No. = reference number, SAH = subarachnoid hemorrhage, VA = vertebral artery, VBA = vertebrobasilar arteries
References
1. Fukuta K, Kudo H, Sasaki M, Kimura J, bin Ismail D, Endo H. Absence of carotid rete mirabile in small tropical ruminants: implications for the evolution of the arterial system in artiodactyls. J Anat. 2007. 210:112–116.
2. Sahin H, Cinar C, Oran I. Carotid and vertebrobasilar rete mirabile: a case report. Surg Radiol Anat. 2010. 32:95–98.
3. Mahadevan J, Batista L, Alvarez H, Bravo-Castro E, Lasjaunias P. Bilateral segmental regression of the carotid and vertebral arteries with rete compensation in a Western patient. Neuroradiology. 2004. 46:444–449.
4. Itoyama Y, Kitano I, Ushio Y. Carotid and vertebral rete mirabile in man--case report. Neurol Med Chir (Tokyo). 1993. 33:181–184.
5. Kim MS, Lee SJ, Lee CH, Park HI. Bilateral segmental absence of the internal carotid artery with rete compensation associated with absence of basilar artery: case report. Surg Neurol. 2006. 65:615–619. discussion 619; author reply 620.
6. Castro S, Abreu P, Azevedo E, Silva ML. A new pattern of arterial rete compensation of segmental basilar agenesis associated with carotid retia mirabilia: a case report (2010: 1b). Eur Radiol. 2010. 20:1024–1028.
7. Li G, Jayaraman MV, Lad SP, Adler J, Do H, Steinberg GK. Carotid and vertebral rete mirabile in man presenting with intraparenchymal hemorrhage: a case report. J Stroke Cerebrovasc Dis. 2006. 15:228–231.
8. Jones RR, Wetzel N. Bilateral carotid vertebrobasilar rete mirabile. Case report. J Neurosurg. 1970. 33:581–586.
9. Koo AH, Newton TH. Pseudoxanthoma elasticum associated with carotid rete mirabile. Case report. Am J Roentgenol Radium Ther Nucl Med. 1972. 116:16–22.
10. Hyogo T, Nakagawara J, Nakamura J, Suematsu K. Multiple segmental agenesis of the cerebral arteries: case report. Neuroradiology. 1996. 38:433–436.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download