INTRODUCTION
MATERIALS AND METHODS
Experimental Model
Dynamic Contrast Enhanced MRI
Histopathological Analysis
Statistical Analysis
RESULTS
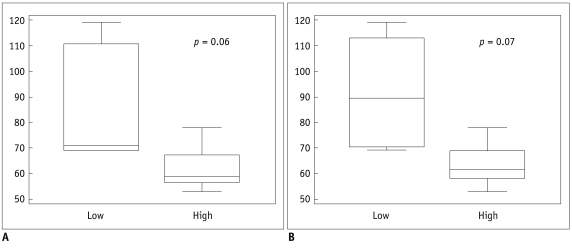 | Fig. 1Box whisker plot of microvascular density evaluating region of interest drawn on hotspots.
Distribution of low and high groups is shown in Ktrans (A) and Kep (B).
|
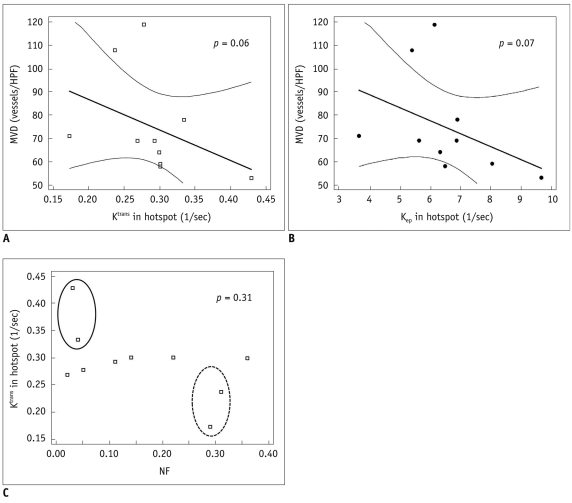 | Fig. 2Representative scatter diagrams showing relationships between microvascular density, necrotic fraction, and dynamic contrast enhanced MRI derived parameters from hotspots.
A, B. Ktrans and microvascular density (Spearman's correlation = -0.61, p = 0.06) (A), Kep and microvascular density (Spearman's correlation = -0.60, p = 0.07) (B). Dashed line indicates 95% confidence interval. C. Necrotic fraction and Ktrans (Spearman's correlation = -0.34, p = 0.31). Solid circle represents group that shows low necrotic fraction and high Ktrans. Dashed circle represents group who showed high necrotic fraction and low Ktrans. MVD = microvascular density, NF = necrotic fraction
|
Table 1
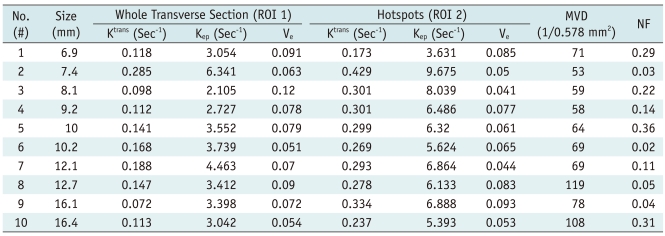
Table 2
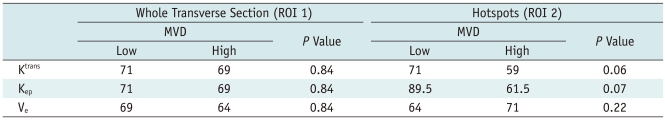
Note.- MVD = microvascular density, Low = group of mice with dynamic contrast enhanced MRI parameter values below median value, High = group of mice with dynamic contrast enhanced MRI parameter values above median value, MVD values are median. P value < 0.05 is considered to be statistically significant.
Table 3

Note.- Correlation coefficient (τ) represents degree of change in two corresponding variables. P value < 0.05 is considered to be statistically significant. *Represents borderline statistical significance. MVD = microvascular density, NF = necrotic fraction, ROI 1 = whole transverse section, ROI 2 = hotspots
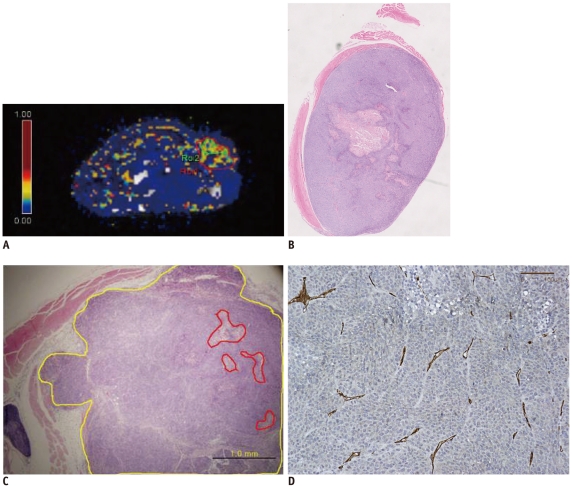 | Fig. 3Pixel-by-pixel analysis of Ktrans in tumor of representative mouse #2 evaluated by dynamic contrast enhanced MRI with Tofts model.
A. Ktrans values of whole tumor (ROI 1) and hotspot (ROI 2) are 0.285/sec and 0.429/sec, respectively. ROI 1 and ROI 2 are marked by red and green solid lines. B. Hematoxylin and Eosin staining of corresponding section of mouse #2 (original magnification, × 2). Necrosis is noted in center of tumor. C. Hematoxylin and Eosin staining includes hotspot area. Tumor necrosis fraction is 0.03 (original magnification, × 40). Yellow line indicates tumor border and red line indicates area of necrosis. D. CD 31 staining of tumor from mouse #2. Microvascular density count is 53 (original magnification, × 200).
|
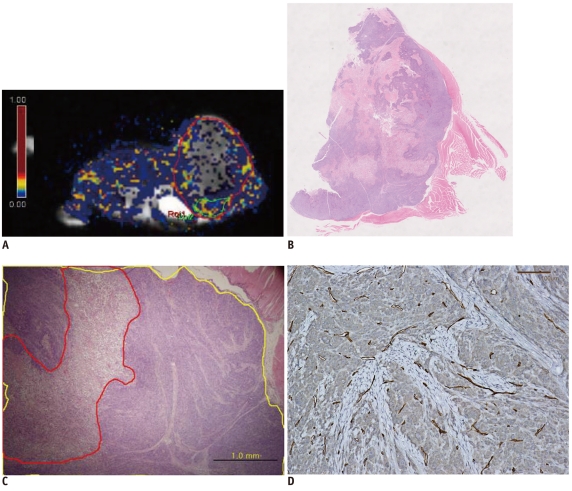 | Fig. 4Pixel by pixel analysis of Ktrans in tumor of another representative mouse (#10).
A. Ktrans values of whole tumors and hotspots were 0.113/sec and 0.237/sec respectively. ROI 1 and ROI 2 are marked by red and green solid lines, respectively. B. Hematoxylin and Eosin staining of corresponding section of mouse #10. Large amount of necrosis is shown (original magnification, × 2). C. Hematoxylin and Eosin staining including hotspot region. Tumor necrotic fraction is 0.31 (original magnification, × 40). Yellow line indicates tumor border and red line indicates area of necrosis. D. CD 31 staining of tissue from mouse #10. Microvascular density count is 108 (original magnification, × 200).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download