Abstract
Objective
To evaluate the effect of temporary stent graft placement in the treatment of benign anastomotic biliary strictures.
Materials and Methods
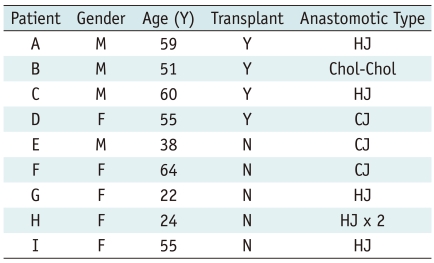
Nine patients, five women and four men, 22-64 years old (mean, 47.5 years), with chronic benign biliary anastomotic strictures, refractory to repeated balloon dilations, were treated by prolonged, temporary placement of stent-grafts. Four patients had strictures following a liver transplantation; three of them in bilio-enteric anastomoses and one in a choledocho-choledochostomy. Four of the other five patients had strictures at bilio-enteric anastomoses, which developed after complications following laparoscopic cholecystectomies and in one after a Whipple procedure for duodenal carcinoma. In eight patients, balloon-expandable stent-grafts were placed and one patient was treated by insertion of a self-expanding stent-graft.
Results
In the transplant group, treatment of patients with bilio-enteric anastomoses was unsuccessful (mean stent duration, 30 days). The patient treated for stenosis in the choledocho-choledochostomy responded well to consecutive self-expanding stent-graft placement (total placement duration, 112 days). All patients with bilio-enteric anastomoses in the non-transplant group were treated successfully with stent-grafts (mean placement duration, 37 days).
Benign biliary strictures are traditionally treated surgically but, due to the significant associated morbidity and mortality (1, 2), less invasive percutaneous and endoscopic methods are often used. Endoscopic treatment may be difficult, particularly in patients with a surgically altered anatomy. Percutaneous access is the remaining alternative and allows repeated balloon dilatations to be performed. If these dilations are unsuccessful, patients often need permanent internal/external biliary catheter drainage which is uncomfortable and requires frequent catheter changes. Bare metal stents have limited long term patency (3-5) due to epithelial hyperplasia and may preclude future surgical procedures (5-7). We present our experience with temporary placement of stent-grafts, with the aim of keeping the strictured segments expanded for a longer period of time, possibly preventing recurrence of the stricture.
Approval for this retrospective analysis was obtained from our Institutional Review Board and informed consent was waived.
Nine patients, five females, and four males aged between 22 and 64 years old (mean, 47.5 years), were treated. Four patients had anastomotic strictures following liver transplantation performed for primary sclerosing cholangitis (n = 2), hepatocellular carcinoma (n = 1), and a large arteriovenous malformation causing heart failure (n = 1). Of the remaining five patients, four had strictures at the bilio-enteric anastomoses that occurred as a result of bile duct injury at laparoscopic cholecystectomy, and one developed a stricture many years after a Whipple procedure for duodenal carcinoma.
All patients required percutaneous placement of permanent internal/external biliary drainage due to stenoses at the surgical anastomoses. In all of these patients (Table 1), the anastomotic strictures failed repeated prolonged (over five minutes) balloon dilations on at least three occasions.
Following informed consent, all procedures were performed under conscious sedation and with administration of the local anesthesia. The existing biliary drainage catheter was removed over a guide wire, and replaced with a 7-9 Fr vascular sheath; cholangiograms were obtained in multiple projections and measurements were performed.
In the eight patients with bilio-enteric anastomoses, 7-9 mm diameter I-cast™ balloon-expandable stent-grafts (Atrium, Hudson, NH) were placed (Fig. 1). The stent-graft was un-mounted from the balloon and cut to the appropriate length (0.5 cm longer than the length of the stricture). Before placement, the stent graft was remounted onto an 8-10 mm diameter, 20-40 mm long balloon, and deployed across the strictures under fluoroscopic guidance.
In the one patient with the choledocho-choledochal anastomosis (post liver transplant), a more flexible 9 mm diameter Viabahn self-expanding stent-graft (W. L. Gore & Associates, Inc. Flagstaff, AZ) was used (Fig. 2). We expected that this stent-graft might be easier to retrieve or dislodge to the bowel, than a balloon-expandable stent-graft.
After placement, an 8 Fr internal/external biliary drain was inserted through the stent graft and was capped. Patients returned for cholangiograms in 2-8 weeks (mean, 33 days). At this time, some of the balloon-expandable stent-grafts had already migrated to the bowel, and the others were pushed into the bowel using a dilation balloon. The self-expanding stent graft had migrated from the anastomosis in the proximal direction, but could be uneventfully removed.
If the absence of a significant stricture was noted on cholangiography, the internal/external biliary drains were converted to external drainage only. The drainage catheters were left above the anastomosis and capped for a period of four to six weeks. At this time, liver function tests were checked and the cholangiography repeated. If good outflow was confirmed, then the catheters were removed. Patients were thereafter followed with liver function tests.
In the presence of insufficient flow through the anastomotic segment, another stent graft was placed for an additional period of time (4-6 weeks).
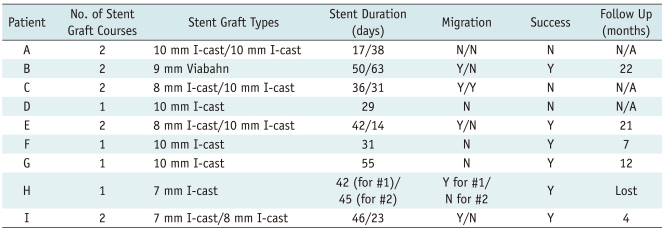
Results are summarized in Table 2.
In the three liver transplant patients with bilio-enteric anastomoses, stent grafts were left in place for an average of 30 days. Two of these patients required repeated stent graft placement. Stent graft migration at the time of the first follow up was noted in one of these three patients. None of these patients could be treated successfully and all required permanent internal/external biliary drainage.
In the transplant patient, with the choledocho-choledochostomy, the first stent graft, left in place for 50 days, migrated in the proximal direction and was removed through a sheath using a snare. A second stent graft remained in place for 63 days and subsequently dislodged to the bowel with a dilation balloon. The treatment was successful and the patient remained stable with normal laboratory values at 22 months follow-up.
Of the five other patients, totaling six bilio-enteric anastomoses (one patient had two separate anastomoses), three were treated with a single stent-graft placement. Of these three, one stent graft had migrated to the bowel at the time of follow up, but the stricture remained properly dilated. The remaining two patients were treated with repeated stent graft placements following the migration of the first one. Each course lasted between 14 and 55 days with a mean of 37 days. Treatment was successful in all these five patients. One patient was lost at follow-up, but the other four remained tube free with stable laboratory values at 4, 7, 12, and 21 months respectively.
An increased number of liver transplants and laparoscopic cholecystectomies during recent years, have been followed by an increased number of patients with benign anastomotic strictures. Surgical revision of these strictures can be difficult and is associated with significant morbidity rate of approximately 25%, and a mortality rate of 2-13% (1, 2), requiring increased length of hospital stay. Furthermore, in a subset of patients with cirrhosis, portal hypertension, and malnutrition, the risks of surgery are even more significant (8, 9). Before surgical revision, endoscopic or percutaneous approaches to treat the stricture are usually attempted. Following the establishment of adequate biliary drainage to relieve the patient's symptoms, repeated balloon dilation of the stricture was attempted. The endoscopic approach is, however, often difficult or not possible in the majority of patients with bilio-enteric anastomoses; and as a result, percutaneous access is most commonly used.
Success rates of balloon dilatation range from 62% to 83% with mean follow-up periods of 24-36 months (10-13). Balloon dilatation appears to be more effective in bile duct strictures related to injuries from laparoscopic cholecystectomies than in anastomotic strictures (14). A patency rate of 71% was achieved in patients with postoperative strictures treated with a combination of balloon dilatation and prolonged drainage with silicon tubes calibrated up to 15 French. Duration of tube placement was one year and the median follow-up time was 34 months (15). The use of cutting balloons also showed encouraging results with long-term patency rates of as much as 90% (16-19).
The efficacy of placement of biliary endoprostheses in patients with benign biliary strictures was evaluated in several studies, but many disadvantages were noted. Notably, endoscopically placed plastic stents had limited patency, could migrate (20), and were usually occluded within three to six months, requiring replacement (21). Percutaneously placed plastic stents also require relatively large access through the liver, thus increasing the possibility of complications.
The use of bare metal stents is associated with long term failure due to mucosal hyperplasia (22) and these stents cannot be removed. Stent grafts have been shown to remain patent for a longer period of time than bare stents in malignant disease (23-25), and may be removed (26, 27). In a series of 44 patients with chronic pancreatitis, gallstone-related strictures, post liver transplant, autoimmune pancreatitis, and primary sclerosing cholangitis, 10 mm stent grafts were placed endoscopically with resolution of the stricture in 83% of cases. The median length of stent treatment was 3.3 months (28). During the removal of these stents, mucosal bleeding was seen in 43% of patients. This was likely related to the stent design, which was tailored to preventing migration. Placement was also technically challenging due to the rigid delivery system of the stent (Viabil, W. L. Gore & Associates, Inc. Flagstaff, AZ).
In a series of 79 patients with benign bile duct strictures, the placement of partially covered metal stents for a median of four months resulted in the resolution of the strictures in 90% of cases (29). Stent migration was seen in 11% of cases and strictures related to mucosal hyperplasia in 8% of cases. Partially covered Wallstents were used in a series of 20 patients with biliary strictures due to chronic pancreatitis (30). Stents were left in place for a median of five months. Persistent stricture resolution at six months after stent removal was seen in 90% of cases. Longer term follow up at a median of 22 months revealed two further patients who needed repeat stent placement and consequently, 80% remained free from strictures. In a series of 14 patients with strictures related to chronic pancreatitis, in whom partially covered Wallstents were placed, seven were found to be complicated by tissue hyperplasia or migration at a median of 22 months (31).
The literature describing the percutaneous placement of self-expanding stent-grafts is limited. Gwon et al. (32) report a series of 29 patients with cholangitis or post-operative strictures who had covered custom-made stents placed percutaneously for a mean of seven weeks. Three patients required additional stent-grafts due to recurrent strictures. At a mean of 28 months after removal of the stent-grafts, the patency rate was 97%. Stent-grafts also prevented the ingrowth of tissue and could be retrieved (32, 33). However, self-expanding stent-grafts are relatively long, making them difficult to place in a short anastomotic stricture. Balloon-expandable stent-grafts as used in our study in all but one patient, can be cut easily to an appropriate length, facilitating its exact deployment.
The findings in our cases suggest that temporary placement of stent grafts has a better effect in non-transplant patients than in the transplant patients. All six of the anastomotic strictures in the non-transplant patients responded to the treatment. In the post-transplant group, none of the patients with bilio-enteric anastomoses responded, however the patient with a choledocho-choledochostomy, did respond. The failure of treatment in bilioenteric anastomoses may be secondary to the altered blood supply after liver transplantation. The ischemia may be less pronounced in the case of a duct-to-duct anastomosis. Secondarily, failure may have resulted from too brief a treatment duration. Short treatment durations were used in our study at the beginning (two weeks), but were successively extended (up to 8 weeks) as patients tolerated the stents being in place well. Better results may have been achieved in the biliary enteric anastomotic transplant patients had the stent grafts been left in place for even longer periods of time.
Stent graft migration was seen in two of our patients from the transplant group and three from the non-transplant group, as has been reported previously (34). However, in our study, stent graft migration did not have an impact on the success of the therapy.
Limitations of our study include the small number of patients, the retrospective nature of the study, and the heterogeneity in the management of patients. In addition, in the cases where stent graft migration occurred, it is impossible to know for how long the device actually remained in the intended position.
We may conclude that treatment was successful in the non-transplant patients, but failed in the liver transplant patients with bilio-digestive anastomoses.
References
1. Röthlin MA, Löpfe M, Schlumpf R, Largiadèr F. Long-term results of hepaticojejunostomy for benign lesions of the bile ducts. Am J Surg. 1998; 175:22–26. PMID: 9445233.

2. Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Sita A. The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Ann Surg. 1996; 224:162–167. PMID: 8757379.

3. Bonnel DH, Liguory CL, Lefebvre JF, Cornud FE. Placement of metallic stents for treatment of postoperative biliary strictures: long-term outcome in 25 patients. AJR Am J Roentgenol. 1997; 169:1517–1522. PMID: 9393155.

4. Yoon HK, Sung KB, Song HY, Kang SG, Kim MH, Lee SG, et al. Benign biliary strictures associated with recurrent pyogenic cholangitis: treatment with expandable metallic stents. AJR Am J Roentgenol. 1997; 169:1523–1527. PMID: 9393156.

5. Hausegger KA, Kugler C, Uggowitzer M, Lammer J, Karaic R, Klein GE, et al. Benign biliary obstruction: is treatment with the Wallstent advisable? Radiology. 1996; 200:437–441. PMID: 8685339.

6. Lopez RR Jr, Cosenza CA, Lois J, Hoffman AL, Sher LS, Noguchi H, et al. Long-term results of metallic stents for benign biliary strictures. Arch Surg. 2001; 136:664–669. PMID: 11387004.

7. Roumilhac D, Poyet G, Sergent G, Declerck N, Karoui M, Mathurin P, et al. Long-term results of percutaneous management for anastomotic biliary stricture after orthotopic liver transplantation. Liver Transpl. 2003; 9:394–400. PMID: 12682893.

8. Deviere J, Cremer M, Baize M, Love J, Sugai B, Vandermeeren A. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self expandable stents. Gut. 1994; 35:122–126. PMID: 8307432.

9. Smits ME, Rauws EA, van Gulik TM, Gouma DJ, Tytgat GN, Huibregtse K. Long-term results of endoscopic stenting and surgical drainage for biliary stricture due to chronic pancreatitis. Br J Surg. 1996; 83:764–768. PMID: 8696734.

10. Misra S, Melton GB, Geschwind JF, Venbrux AC, Cameron JL, Lillemoe KD. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg. 2004; 198:218–226. PMID: 14759778.

11. Vos PM, van Beek EJ, Smits NJ, Rauws EA, Gouma DJ, Reeders JW. Percutaneous balloon dilatation for benign hepaticojejunostomy strictures. Abdom Imaging. 2000; 25:134–138. PMID: 10675453.

12. Schumacher B, Othman T, Jansen M, Preiss C, Neuhaus H. Long-term follow-up of percutaneous transhepatic therapy (PTT) in patients with definite benign anastomotic strictures after hepaticojejunostomy. Endoscopy. 2001; 33:409–415. PMID: 11396758.

13. Kim JH, Lee SK, Kim MH, Song MH, Park DH, Kim SY, et al. Percutaneous transhepatic cholangioscopic treatment of patients with benign bilio-enteric anastomotic strictures. Gastrointest Endosc. 2003; 58:733–738. PMID: 14595311.

14. Ramos-De la Medina A, Misra S, Leroy AJ, Sarr MG. Management of benign biliary strictures by percutaneous interventional radiologic techniques (PIRT). HPB (Oxford). 2008; 10:428–432. PMID: 19088929.

15. Glas L, Courbière M, Ficarelli S, Milot L, Mennesson N, Pilleul F. Long-term outcome of percutaneous transhepatic therapy for benign bilioenteric anastomotic strictures. J Vasc Interv Radiol. 2008; 19:1336–1343. PMID: 18725096.

16. Atar E, Bachar GN, Bartal G, Mor E, Neyman H, Graif F, et al. Use of peripheral cutting balloon in the management of resistant benign ureteral and biliary strictures. J Vasc Interv Radiol. 2005; 16:241–245. PMID: 15713925.

17. Saad WE, Saad NE, Davies MG, Lee DE, Patel NC, Sahler LG, et al. Transhepatic balloon dilation of anastomotic biliary strictures in liver transplant recipients: the significance of a patent hepatic artery. J Vasc Interv Radiol. 2005; 16:1221–1228. PMID: 16151063.

18. Kakani NK, Puckett M, Cooper M, Watkinson A. Percutaneous transhepatic use of a cutting balloon in the treatment of a benign common bile duct stricture. Cardiovasc Intervent Radiol. 2006; 29:462–464. PMID: 16447007.

19. Sheridan JS, Maclennan AC. Percutaneous transhepatic use of a cutting balloon in the treatment of a benign common bile duct stricture. Cardiovasc Intervent Radiol. 2007; 30:346. PMID: 17200895.

20. Eickhoff A, Jakobs R, Leonhardt A, Eickhoff JC, Riemann JF. Endoscopic stenting for common bile duct stenoses in chronic pancreatitis: results and impact on long-term outcome. Eur J Gastroenterol Hepatol. 2001; 13:1161–1167. PMID: 11711771.

21. Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001; 54:162–168. PMID: 11474384.

22. Dumonceau JM, Devière J, Delhaye M, Baize M, Cremer M. Plastic and metal stents for postoperative benign bile duct strictures: the best and the worst. Gastrointest Endosc. 1998; 47:8–17. PMID: 9468417.

23. Bezzi M, Zolovkins A, Cantisani V, Salvatori FM, Rossi M, Fanelli F, et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol. 2002; 13:581–589. PMID: 12050298.

24. Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, et al. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53:729–734. PMID: 15082593.

25. Han YM, Kwak HS, Jin GY, Lee SO, Chung GH. Treatment of malignant biliary obstruction with a PTFE-covered self-expandable nitinol stent. Korean J Radiol. 2007; 8:410–417. PMID: 17923784.

26. Kahaleh M, Tokar J, Le T, Yeaton P. Removal of self-expandable metallic Wallstents. Gastrointest Endosc. 2004; 60:640–644. PMID: 15472699.

27. Cahen DL, Rauws EA, Gouma DJ, Fockens P, Bruno MJ. Removable fully covered self-expandable metal stents in the treatment of common bile duct strictures due to chronic pancreatitis: a case series. Endoscopy. 2008; 40:697–700. PMID: 18704837.

28. Mahajan A, Ho H, Sauer B, Phillips MS, Shami VM, Ellen K, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009; 70:303–309. PMID: 19523620.

29. Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc. 2008; 67:446–454. PMID: 18294506.

30. Behm B, Brock A, Clarke BW, Ellen K, Northup PG, Dumonceau JM, et al. Partially covered self-expandable metallic stents for benign biliary strictures due to chronic pancreatitis. Endoscopy. 2009; 41:547–551. PMID: 19533560.

31. Cantù P, Hookey LC, Morales A, Le Moine O, Devière J. The treatment of patients with symptomatic common bile duct stenosis secondary to chronic pancreatitis using partially covered metal stents: a pilot study. Endoscopy. 2005; 37:735–739. PMID: 16032492.

32. Gwon DI, Shim HJ, Kwak BK. Retrievable biliary stent-graft in the treatment of benign biliary strictures. J Vasc Interv Radiol. 2008; 19:1328–1335. PMID: 18662891.

33. Petersen BD, Timmermans HA, Uchida BT, Rabkin JM, Keller FS. Treatment of refractory benign biliary stenoses in liver transplant patients by placement and retrieval of a temporary stent-graft: work in progress. J Vasc Interv Radiol. 2000; 11:919–929. PMID: 10928533.

34. Eickhoff A, Jakobs R, Leonhardt A, Eickhoff JC, Riemann JF. Self-expandable metal mesh stents for common bile duct stenosis in chronic pancreatitis: retrospective evaluation of long-term follow-up and clinical outcome pilot study. Z Gastroenterol. 2003; 41:649–654. PMID: 12908456.
Fig. 1
Stent graft in postsurgical benign biliary stricture.
A. 46-year-old male patient following liver transplant with tight stenosis (arrow) at choledocho-choledochostomy. B. Placement of 9 mm self-expanding stent (Viabahn) across the stricture (arrow). C. Two months after placement, stent graft is pushed into bowel and there is free flow of contrast from biliary tree to bowel (arrow).
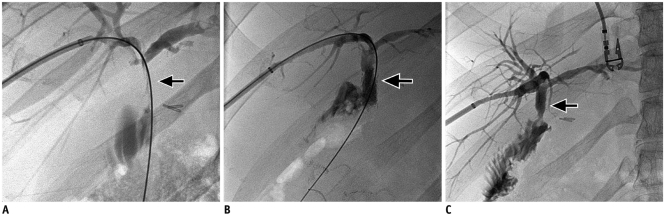
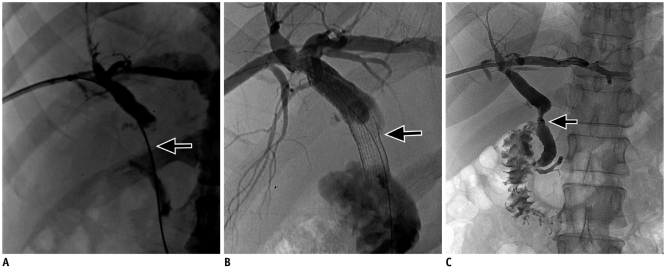
Fig. 2
Stent graft in postsurgical benign biliary stricture.
A. Tight stricture (arrow) at hepaticojejunostomy in 38-year-old patient with bile duct injury following cholecystectomy. B. Placement of 8 mm stent-graft (I-cast) across stricture (arrow). C. Stent is pushed into bowel after 10 weeks, revealing patent anastomosis with free flow of contrast on subsequent cholangiogram (arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download