Abstract
Bone metastasis from a spinal cord astrocytoma has been reported only twice in the English medical literature. It is generally known that bone metastasis is found after the initial diagnosis with/without intervening surgery rather than being found at the time of the diagnosis of astrocytoma. The purpose of this article is to report for the first time a case of concurrent bone metastasis from a spinal cord astrocytoma at the time of diagnosing the spinal cord astrocytoma.
While astrocytomas are a relatively common glial neoplasm of the central nervous system, only 3% of the cases are found in the spinal cord. The incidence of spinal cord astrocytoma is 0.8 to 2.5 out of every 100,000 people per year (1). Moreover, systemic metastasis from a spinal cord astrocytoma is very rare even when the tumor grade is pathologically high (2, 3). The mechanism of extraneural metastasis of the tumors of the central nervous system, including astrocytoma, is frequently explained as direct access of tumor to the extrameningeal tissue via the dural vessels. Lymphatic spread through the invasion of the extrameningeal tissue is also possible despite the absence of lymphatics in the central nervous system (4). In the context of extrameningeal tissue invasion, systemic metastasis is reportedly triggered by surgery (4). However, there has been no report demonstrating concurrent bony metastasis at the time of initial diagnosis of a spinal cord astrocytoma. We experienced a case of spinal cord astrocytoma with concurrent bone metastasis at the time of the initial diagnosis. Although there are reports on metastasis of a spinal cord astrocytoma to bone, they did not detail the imaging findings (2, 3). Hence, the purpose of this article is to present the imaging findings of concurrent bone metastasis from a spinal cord astrocytoma at the time of the initial diagnosis, and these particular imaging findings have never been reported in the English medical literature.
A 50-year-old male patient was referred to our institution for evaluation of a known spinal cord mass. He presented with left leg weakness and low back pain for the previous 6 months. On the lumbar spine magnetic resonance (MR) images taken with a 1.5 Tesla (T) MR scanner and in the hospital that referred the patient to us, about a 2 cm sized mass that arose from the conus medullaris was found at the level of the T12 to L1 vertebrae. Compared with the adjacent muscles, the signal intensity of the mass was heterogeneously hyperintense on the fat suppressed T2-weighted image (T2WI) (Fig. 1A) and it was isoinstense on the T1-weighted image (T1WI) (Fig. 1B). The mass showed multifocal nodular enhancement on the fat-suppressed T1WI obtained after the intravenous injection of gadolinium contrast agent (Fig. 1C). Although they were overlooked during the initial assessment of these MR images from the referring hospital, multiple bony lesions were present in the thoracolumbar vertebral bodies and sacrum. Compared with the adjacent muscle, the lesions were heterogeneously hyperintense on the fat suppressed T2WI (Fig. 1A) and they were hypointense on the T1WI (Fig. 1B). The bony lesions showed heterogeneous enhancement on the fat suppressed T1WI obtained after the intravenous injection of gadolinium compound (CE T1WI) (Fig. 1C). Eight days after the first MR imaging, the intramedullary mass was partially removed for the purpose of pathologic confirmation and to halt further neurologic impairment. The final pathologic examination revealed the anaplastic astrocytoma (Fig. 1D). For the remaining primary mass, the patient received radiation therapy to the spinal canal at the level of the T12 through the L1 vertebral bodies with a dosage of 5400 cGy.
The follow-up MR imaging was performed 60 days after the surgery to assess the postsurgical and radiotherapy status of the spinal cord. The MR images revealed a shrunken primary mass of the spinal cord. However, the bony lesions of the entire vertebral column were found to be worsened and they had become heterogeneous, and bone metastasis was considered to have already happened at that time (Fig. 1E-G). Subsequently, computed tomography (CT) of the chest, abdomen and pelvis was performed right after the follow-up MR imaging for the further evaluation of distant metastasis. Matching all the bony lesions depicted on the MR images with those seen on the CT images, the lesions demonstrated hyperdense attenuation, suggesting osteoblastic metastasis (Fig. 1H). A whole body bone scan and PET-CT (positron emission tomography-computed tomography) were also sequentially performed within the next two days after the CT examinations. On the whole body bone scan images obtained from a dual head gamma camera (Millennium VG, GE Medical Systems, Milwaukee, WI), multiple foci of heterogeneously increased bony uptakes were detected in the thoracolumbar spine, the bilateral rib cages and the pelvic bones, which were thought to be consistent with the multiple bony lesions noted on CT and MRI (Fig. 1I). On the whole body 18F-fluorodeoxyglucose (FDG) PET-CT using a Biograph 40 (Siemens/CTIMI, Knoxville, TN), among all the osteoblastic lesions, focally increased FDG uptake was noted only on the T11 vertebral body (the SUV 2.19). No extraskeletal metastatic focus was observed on PET-CT (Fig. 1J). For an accurate pathologic diagnosis of the bony lesions, CT-guided percutaneous biopsy was performed with targeting the lesion of the T11 vertebral body based on the findings of PET-CT. However, the biopsy revealed a non-satisfactory result because the amount of specimen was not sufficient for making a confirmative diagnosis. Hence, CT-guided localization was carried out using a guide pin and then a subsequent open bone biopsy was done, instead of a percutaneous biopsy, for the iliac bone (Fig. 1K). A bone block measuring 4 × 1 × 2 cm was obtained, and the pathologic result from the specimen revealed metastatic anaplastic astrocytoma (Fig. 1L).
The patient had undergone 2 cycles of chemotherapy with the use of carboplatin and vincristine before he was found to have bone metastasis. However, after the diagnosis of bone metastasis, the chemotherapy regimen was changed into temozolomide, and cisplatin was also administered to the patient during one of the cycles. After the last session of chemotherapy, the patient was lost to follow up.
Systemic metastasis involving the bone from a spinal cord astrocytoma is very rare partly because spinal cord astrocytoma is relatively rare per se: spinal cord astrocytoma makes up only 3% of all astrocytomas of the central nervous system (1). It is also because extraneural metastases from central nervous system astrocytomas involving the bone are quite rare, and particularly when the spinal cord is the primary site (2). Hence, various clinical aspects of systemic metastasis from a spinal cord astrocytoma have not been well elucidated.
There have been many reports about systemic metastasis of astrocytoma, albeit most of the reports have been about metastasis from intracranial astrocytomas, which is unlike our case. However, with all the reports, there has been no report that showed the imaging findings, with using various imaging modalities, of bony metastasis from an astrocytoma, including the imaging findings of radiography, CT, MRI, bone scintigraphy and PET-CT. Among the many reports about bony metastasis from astrocytoma with the imaging findings and regardless whether the location of the primary tumor was intracranial or extracranial, only six reports with six cases included the MRI findings of metastatic lesions (4-9). The most common MRI finding of the bony metastatic lesions from these reports was enhancement on the contrast enhanced T1WI, but the signal intensity of the lesions on T1WI and T2WI was diverse or nonspecific (4-9). These metastatic foci were presented as either osteoblastic or osteolytic lesions on the plain radiography or CT images. Four cases among them presented the scintigraphic findings of the bony metastatic lesions, which all showed, except one case, increased bony uptake (4, 5, 8, 9). The metastatic lesions of our case also showed nonspecific signal intensity and enhancement on the gadolinium enhanced T1WI. Particularly, the osteoblastic lesions of our case showed heterogeneous signal intensity on the T2WI, which is presumed to be associated with mineralization of the lesions. In our case, not all the bone lesions depicted on MR imaging showed bony uptake on scintigraphy, which shows the need of MR imaging for assessing bone metastasis even though the metastatic lesions were osteoblastic. Regarding the mismatch of the findings (the positive MR imaging findings and the negative findings on the bone scan at the corresponding locations), Taoka et al. (10) explained that early vertebral metastases tend to be small and located in the medullary cavity with abundant vascularity. They stated that MR imaging is useful for the detection of such early intramedullary metastatic lesions (10). No previous studies have reported on the PET-CT findings of metastatic bone tumors from astrocytoma. Only the lesion located at the T11 vertebral body in our case showed increased FDG uptake, whereas the FDG uptake was not strong for the remaining bone marrow lesions. We tentatively believe that the routine use of PET-CT, as a screening or follow-up tool for bone metastasis, cannot be advocated for the case of a spinal cord astrocytoma.
There have been only two old reports about spinal cord astrocytomas with accompanying bone metastases (2, 3). Both the cases were high grade astrocytoma like our case. The first report in 1971 was a case of glioblastoma multiforme that originated from the lower thoracic spinal cord with bone metastases (3). The second report in 1984 was a case of spinal cord glioblastoma with disseminated bone metastases to the ribs, humeri, scapulae, pelvis and right femur (2). Our case is the third case of high grade spinal cord astrocytoma that metastasized to the bone, but it is worthy of notice in two aspects.
Above all, this is the first report where bone metastasis from a spinal cord astrocytoma was present at the time of the initial diagnosis. In the aforementioned two cases, the bony metastases were found after the initial diagnosis. Those cases also showed intracranial astrocytomas; therefore, it is not completely certain whether the spinal cord was the primary focus. As for intracranial astrocytoma, concurrent metastasis has been reported just once in a case report (4). Additionally, our case demonstrated the comprehensive imaging findings, including CT, MRI, scintigraphy and PET-CT scans.
To summarize, multiple bony metastases from spinal cord astrocytomas are extremely rare, but they are definitely within the range of possibility. For patients with high grade spinal cord astrocytomas with any subtle abnormality seen in the imaging finding of the bones, metastatic bone lesions should be scrutinized without hesitation. As for the imaging findings, the bony metastatic lesions can be osteolytic or osteoblastic on radiographs and CT. On MRI, the lesions can be detected earlier as heterogeneous hyperintense lesions on T2WI and heterogeneously enhanced lesions on the contrast enhanced T1WI. On scintigraphy and PET-CT scanning, the lesions can show variable uptake. If we detect bony lesions through any imaging modalities, then we should consider a diagnosis of bone metastasis. Pathologic confirmation via a bone biopsy may be necessary for a more accurate pathologic evaluation.
Figures and Tables
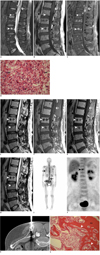 | Fig. 1Bone metastasis identified concurrently with primary spinal cord astrocytoma.
A-C. Fat suppressed T2-weighted image shows heterogeneous hyperintense mass of spinal cord (arrows) at level of T12 and L1 vertebrae (A), T1-weighted image shows isointensity of this mass (B), and fat-suppressed contrast enhanced T1-weighted image shows multifocal nodular enhancement (C). Fat suppressed T2-weighted image shows heterogeneous hyperintense multiple metastatic bone marrow lesions (arrowheads) (A), T1-weighted image shows hypointensity of these lesions (B) and fat suppressed contrast enhanced T1-weighted image shows heterogeneous enhancement (C). D. Microscopic examination (Hematoxylin & Eosin staining, original magnification: × 400) reveals high cellularity and nuclear pleomorphism (arrows) in fibrillary background without microvessel proliferation or tumor necrosis, which all suggests anaplastic astrocytoma. E-H. T2-weighted image shows aggravated multiple bone marrow lesions (arrowheads) with variable signal intensity (E), T1-weighted image shows hypointensity of these lesions (F) and contrast enhanced T1-weighted image shows variable contrast enhancement (G). These lesions, which correspond to those of MR images, are depicted as osteoblastic lesions on sagittal reformatted CT images of spine (H). I, J. Scintigraphy (I) and whole body 18F-FDG PET-CT (J) images of patient. Whole body bone scan (I) shows multiple foci of heterogeneously increased bony uptake (arrows) at thoracolumbar spine, rib cage and pelvic bones, which are thought to be consistent with multiple bony lesions seen on CT and MRI. Whole body FDG PET image (J) shows increased focal FDG uptake (arrowhead) of vertebral body, which is clearly visualized as being located in T11 vertebral body on fusion image of PET-CT. Extraskeletal FDG uptake is not observed. K, L. CT-guided localization using guide pin for subsequent open bone biopsy (K) and photomicrograph of surgical specimen from excised bone (L). CT image obtained with localization clearly shows guide pin whose tip is located at center of iliac bone, which contains osteoblastic lesions (K). Histologic examination of specimen (Hematoxylin & Eosin staining, original magnification × 100) reveals bone marrow and metastatic tumor (T). Bone marrow (BM) is infiltrated by metastatic tumor cells (arrowheads) that show histologic findings identical to those of spinal cord tumor.
|
References
1. Roonprapunt C, Houten JK. Spinal cord astrocytomas: presentation, management, and outcome. Neurosurg Clin N Am. 2006. 17:29–36.
2. Newman RP, Schaefer EJ, Thomas CB, Oldfield EH. Abetalipoproteinemia and metastatic spinal cord glioblastoma. Arch Neurol. 1984. 41:554–556.
3. Eade OE, Urich H. Metastasising gliomas in young subjects. J Pathol. 1971. 103:245–256.
4. Fabi A, Vidiri A, Carapella C, Pace A, Occhipinti E, Caroli F, et al. Bone metastasis from glioblastoma multiforme without central nervous system relapse: a case report. Anticancer Res. 2004. 24:2563–2565.
5. Beauchesne P, Soler C, Mosnier JF. Diffuse vertebral body metastasis from a glioblastoma multiforme: a technetium-99m Sestamibi single-photon emission computerized tomography study. J Neurosurg. 2000. 93:887–890.
6. Rajagopalan V, El Kamar FG, Thayaparan R, Grossbard ML. Bone marrow metastases from glioblastoma multiforme--A case report and review of the literature. J Neurooncol. 2005. 72:157–161.
7. Utsuki S, Tanaka S, Oka H, Iwamoto K, Sagiuchi T, Fujii K. Glioblastoma multiforme metastasis to the axis. Case report. J Neurosurg. 2005. 102:540–542.
8. Mihara F, Ikeda M, Rothman MI, Numaguchi Y, Kristt D. Vertebral body metastasis of glioblastoma multiforme with epidural mass formation. Contrast-enhanced MRI study. Clin Imaging. 1994. 18:386–389.
9. Myers T, Egelhoff J, Myers M. Glioblastoma multiforme presenting as osteoblastic metastatic disease: case report and review of the literature. AJNR Am J Neuroradiol. 1990. 11:802–803.
10. Taoka T, Mayr NA, Lee HJ, Yuh WT, Simonson TM, Rezai K, et al. Factors influencing visualization of vertebral metastases on MR imaging versus bone scintigraphy. AJR Am J Roentgenol. 2001. 176:1525–1530.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download