Abstract
Pacemakers and implantable cardioverter defibrillators (ICDs) are being increasingly employed in patients suffering from cardiac rhythm disturbances. The principal objective of this article is to familiarize radiologists with pacemakers and ICDs on chest radiographs and CT scans. Therefore, the preferred lead positions according to pacemaker types and anatomic variants are introduced in this study. Additionally, the imaging features of incorrect lead positions and defects, as well as complications subsequent to pacemaker implantation are demonstrated herein.
Permanent pacemakers and implantable cardioverter defibrillators (ICD), also known as cardiovascular implantable electronic devices (CIED), are being increasingly employed for the treatment of patients suffering from various cardiac arrhythmias. Overall, there are 4.5 million people worldwide with a CIED (1). CIED implantation has been associated with both acute and delayed complications. Following pacemaker implantation, up to 22% of patients may evidence abnormalities on post-interventional chest radiographs (2). Chest radiographs are helpful in evaluating the lead position and integrity following implantation, and are also helpful in identifying CIED-associated complications. Chest radiographs should be acquired in both posterior-anterior and lateral projection using the hard beam technique (tube potential ≥ 110 kV). Chest CT scans (e.g. tube potential 110 kVp; tube current 100 mAs) may provide more detailed information regarding thoracic complications and CIED. If necessary, ECG-triggered cardiac CT can visualize exact lead positions and potential lead perforations.
Pacemaker systems are generally composed of a pulse generator and one or more leads (Fig. 1). Pulse generators consist of a titanium body which contains a lithium iodine cell. The pulse generator is preferentially implanted in a subcutaneous pocket at the pectoralis muscle, although other locations such as the abdomen, especially in pediatric patients, can also be used.
Pacemaker leads consist of metal conductors with a silicone or polyurethane isolation. Leads are preferably inserted via the cephalic or subclavian vein, but can also be inserted via the jugular or femoral vein. Generic codes of the North American Society of Pacing and the British Pacing and Electrophysiology Group describe the mode of operation of these devices, which generally consist of three positions describing the chambers that are paced (first letter) and sensed (second letter) and the response to sense events (third letter). A fourth position is used if the pacemaker adjusts the pacing rate in response to exercise (R). A fifth position can be used as well, to describe the chambers in which multisite pacing is delivered (Table 1).
Knowledge of the typical lead positions for each pacemaker and ICD type is essential for correct interpretation of chest radiographs (Tables 2, 3).
Chronic atrial fibrillation with ventricular bradycardia is the principal indication for VVI pacemakers, which consist of a single ventricular lead (Fig. 2). In the case of ventricular pacing, the atrium might be exposed to a non-physiological retrograde stimulation, which may result in atrioventricular dyssynchrony with reversed atrial blood flow and abnormal atrial pressure waves ("pacemaker syndrome") (3).
The most frequent indication for an AAI pacemaker is a sick sinus syndrome. AAI pacemakers consist of a single atrial lead (Fig. 3). An intact AV conduction is required for the application of this type of pacemaker. The main disadvantage of this pacing modality is an increased risk of atrioventricular blockade in patients with sick sinus syndrome.
DDD pacemakers consist of two leads--one in the right atrium and one in the right ventricle (Fig. 4). The main indication for a DDD pacemaker is symptomatic failure in AV conduction (AV block °II and °III) in combination with sick sinus syndrome. The majority of newly implanted pacemakers are DDD pacemakers (4).
Severe heart failure with left-sided intraventricular conduction delay is the most common indication for a biventricular pacemaker. Pacemaker electrodes are placed in the right atrium, the right ventricle, and the left ventricle (via the coronary sinus) for the synchronization of atrial and ventricular contractions, which improves the cardiac ejection fraction (5) (Fig. 5).
Implantable cardioverter defibrillators (ICD) are employed for primary and secondary prophylaxis of sudden cardiac death from ventricular tachycardia in high-risk patients (Fig. 6). ICDs are mainly implanted in patients with a history of ventricular fibrillation and sustained ventricular tachycardia. A meta-analysis of secondary prevention ICD trials demonstrated a significant (50%) reduction in arrhythmic death (6). ICDs can also have a post-shock pacing function. Depending on the pacing functions, additional leads can be found in the right atrium and branches of the coronary sinus at the left ventricle.
Persistent left superior vena cava (PLSVC) is the most common venous anomaly of the thorax and can be seen in 0.5-2% of the general population and up to 10% of patients with congenital heart diseases (7). Venous blood from the PLSVC is generally directed to the right atrium via a large coronary sinus (Fig. 7). Pacemaker implantation has been reported to be more challenging in patients with a PLSVC, particularly in the absence of a bridging vein between the PLSVC and the right superior vena cava (7).
Following cardiac surgery, up to 21% of patients may require a permanent pacemaker (8). The ventricular lead can be guided through a reconstructed tricuspidal valve to the right ventricle (Fig. 8). In patients having undergone a mechanical tricuspidal valve replacement, the ventricular lead should not traverse the mechanical valve. The ventricular lead can be placed at the right ventricle via the coronary sinus as an alternative to epimyocardial leads (Fig. 9).
Transposition of the great arteries (TGA) accounts for 5-7% of congenital heart diseases. Arterial switch surgery is currently regarded as the therapy of choice in patients with TGA. However, the Mustard procedure was generally performed in the past to direct venous blood via a "baffle" to the left atrium and oxygenated blood from the pulmonary veins to the right atrium (Fig. 10). In up to 65% of patients having undergone Mustard surgery, cardiac arrhythmias might develop, possibly requiring ICD and/or pacemaker implantation (9).
According to recent literature, the overall incidence of short-term implantation-related complications has been reported to be as high as 12% (10) (Table 4). Aside from pocket hematoma, which may not be visible on chest radiograph, lead dislodgement and unsatisfactory lead positions are the most common complications, and may account for up to 27% of all complications (10-12) (Figs. 11, 12, 13, 14).
Twiddler's syndrome describes a rare condition of pacemaker malfunction. Patients cause lead dislodgment by often unconscious manipulation of the pacemaker generator in its subcutaneous pocket (15). Twiddler's syndrome can be readily diagnosed on chest radiograph and generally appears within the first year after implantation (Fig. 15). Besides female gender, obesity and older age, dementia is generally regarded as the most important risk factor (15).
Lead fractures can occur at any timepoint following implantation. The incidence ranges between 1 to 4% (16, 17). Lead fractures may appear as a complete disruption of the lead or as subtle damage to the lead. The most common sites are the connection point to the generator and the area lateral to the subclavian venous entry site, where the lead might be compressed between the clavicle and the first rip (subclavian crush syndrome) (18) (Figs. 16, 17).
Symptomatic heart perforation in the short-term after pacemaker implantation is rare (0.4-1%) and may be associated with pericardial effusions, which may result in cardiac tamponade (19, 20). Additionally, a handful of case reports have described contralateral pneumothorax as a result of myocardial and pleural lead perforation (21). Delayed asymptomatic perforation has been described to occur in 15% of patients. Asymptomatic atrial perforation occurs more frequently than ventricular perforation (22) (Figs. 18, 19, 20, 21).
The postcardiotomy syndrome (PPS), also referred to as Dressler syndrome, has been widely described after cardiac surgery. It generally manifests 1-3 months after surgery with the most clinical symptoms of fever, chest pain, and pericardial effusion. The underlying pathophysiology has yet to be thoroughly understood, although an autoimmune response to disturbance of the pericardial integrity is believed to play an important role in this phenomenon. In rare cases, PPS has also been reported following pacemaker implantation in adults and children (23) (Fig. 22).
Figures and Tables
Fig. 1
Cardiovascular implantable electronic devices consists of one or more leads (black arrows) connected to pulse generator (white arrow).

Fig. 2
75-year-old female patient with single lead ventricular pacemaker (VVI). Posteroanterior (PA) and lateral chest radiographs show preferred tip position in apex of right ventricle (arrows).
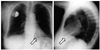
Fig. 3
72-year-old female patient with single lead atrial pacemaker (AAI). PA and lateral chest radiographs show preferred tip position in right atrial appendage (arrows). Note that lead typically curves superiorly and anteriorly into atrial appendage.
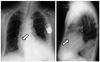
Fig. 4
57-year-old female patient with two-lead bichamber pacemaker (DDD). Preferred tip position is right atrial appendage (white arrows) and apex of right ventricle (black arrows), as seen on PA and lateral chest radiographs.
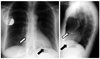
Fig. 5
69-year-old male patient with biventricular pacemaker (CRT). PA and lateral chest radiographs show preferred tip position in right atrial appendage (white arrows), apex of right ventricle (black arrows), and in posterior coronary vein at left ventricle (arrowheads).
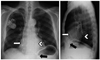
Fig. 6
67-year-old patient with implantable cardioverter defibrillator (ICD). PA and lateral chest radiographs demonstrate preferred tip location in apex of right ventricle (white arrows). Implantable cardioverter defibrillator leads contain one or two coils (*) to enable delivery of energy to myocardium. Proximal coil typically resides within superior vena cava. Note that lead tracks along base of right atrium, curves superiorly as it transverses tricuspid valve, and then turns inferiorly as it enters right ventricle.
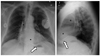
Fig. 7
77-year-old male patient with persistent left superior vena cava following implantation of dual-chamber implantable cardioverter defibrillator. Right atrium and ventricle are reached via coronary sinus. Note superior vena cava lead (arrows) in persistent left superior vena cava.
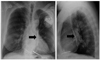
Fig. 8
53-year-old male patient with DDD pacemaker following mitral and tricuspidal valve annuloplasties. PA and lateral chest radiographs show that right ventricular lead (arrowheads) can traverse tricuspid annuloplasty (arrows).
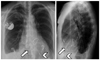
Fig. 9
69-year-old female patient after mechanical tricuspidal valve replacement (TVR, arrows). In this case, placement of ventricular lead (arrowhead) should be via coronary sinus, as seen on PA and lateral chest radiographs.
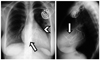
Fig. 10
21-year-old male patient with dual-chamber implantable cardioverter defibrillator following Mustard procedure to correct for transposition of great arteries. Scheme (B) and CT scan performed prior to implantable cardioverter defibrillator placement (C-E) show cardiovascular anatomy. Baffle was constructed to direct venous blood to left atrium (B). Oxygenated blood from pulmonary veins is directed to right atrium (RA) and from there to systemic right ventricle (RV) (B-E). PA and lateral chest radiographs (A) demonstrate leads that course from left brachiocephalic vein into anatomical left atrium (functional right atrium, arrowhead) and into anatomical left ventricle (LV) (functional right ventricle).
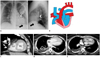
Fig. 11
77-year-old male patient with dual-chamber implantable cardioverter defibrillator. Initial PA and lateral chest radiographs (A) show displacement of atrial lead (white arrows) into right ventricle. After revision, PA and lateral radiographs (B) show correct lead position in right atrium (black arrows). Note epimyocardial lead over right ventricle (*).
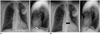
Fig. 12
61-year-old male patient with atrial septal defect following implantation of DDD pacemaker. PA and lateral chest radiographs demonstrate correct position of right atrium lead, whereas ventricular lead curves superiorly and to left just above level of right atrial lead (arrowhead). This course is typical for malpositioned lead traversing from right atrium to left atrium via atrial septal defect and then into left ventricle (arrows).
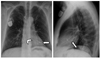
Fig. 13
77-year-old male patient with DDD pacemaker. PA chest radiograph shows superiorly facing ventricular lead tip, which is suggestive of malposition in coronary sinus (arrow in PA radiograph). Malposition in coronary sinus over left ventricle is confirmed on lateral radiograph (arrow).

Fig. 14
35-year-old male patient after implantation of DDD pacemaker. PA and lateral chest radiographs show loop of ventricular lead in right ventricular outflow tract (arrows), which caused insufficiency of pulmonary valve.
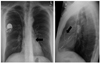
Fig. 15
42-year-old male patient with Twiddler syndrome. Initial PA radiograph shows preferred lead position and generator configuration of single lead ventricular pacemaker (VVI) (A). Several months later, winding of pacemaker lead around generator can be seen on PA chest radiograph (B), causing dislodgement of ventricular lead (arrow).

Fig. 16
52-year-old male patient with DDD pacemaker. PA chest radiograph shows fracture (arrowhead) of ventricle lead and insulation breach (arrow) of atrial lead in subclavian vein adjacent to clavicle ("subclavian crush syndrome").
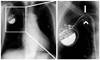
Fig. 17
59-year-old male with single ventricular lead pacemaker. PA chest radiograph discloses lead fracture and dislodgement of lead fragment into hepatic vein.
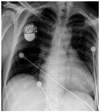
Fig. 18
82-year-old female patient with DDD pacemaker. Sagittal (A) and transverse (B) CT reconstructions show perforation of atrial pacemaker lead (arrow) with development of significant hemopericardium (*).
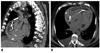
Fig. 19
59-year-old male patient following DDD pacemaker implantation. Hematopneumothorax (arrows) is seen on PA and lateral chest radiographs on contralateral side of pacemaker generator (A). Additional CT scan confirms hematopneumothorax (B, *) and shows perforation of atrial lead (arrowheads in B, C). (Courtesy of Dr. Keske, Gelsenkirchen, Germany).

Fig. 20
84-year-old female patient with DDD pacemaker. Perforation of atrial pacemaker lead is seen on PA chest radiograph (arrow).
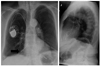
Fig. 21
84-year-old female patient with DDD pacemaker suffering from diaphragmatic stimulation. PA and lateral chest radiographs (A) indicate correct lead positions. However, asymptomatic perforation of atrial (arrow) and ventricular (arrowhead) lead is seen on contrast-enhanced CT scans (B, C). Note upside-down stomach (*).

Fig. 22
61-year-old female suffering from postcardiotomy syndrome. PA chest radiograph of 61-year-old female patient prior to pacemaker implantation shows regular heart size (A). Correct lead position is seen immediately following implantation of DDD pacemaker (B). Three months later, patient suffered from fever and poor cardiac function. Massive pericardial effusion was observed seen on PA chest radiograph (C), which was attributed to postcardiotomy syndrome.
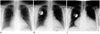
Table 1
Generic Codes Describe Mode of Operation of Pacemaker Systems and May Consist of 3-5 Letters

References
1. Borek PP, Wilkoff BL. Pacemaker and ICD leads: strategies for long-term management. J Interv Card Electrophysiol. 2008. 23:59–72.
2. Grier D, Cook PG, Hartnell GG. Chest radiographs after permanent pacing. Are they really necessary? Clin Radiol. 1990. 42:244–224.
3. Heldman D, Mulvihill D, Nguyen H, Messenger JC, Rylaarsdam A, Evans K, et al. True incidence of pacemaker syndrome. Pacing Clin Electrophysiol. 1990. 13:1742–1750.
4. Markewitz A. [Yearly report for 2007 of the German Pacemaker registry]. Herzschrittmacherther Elektrophysiol. 2008. 19:195–223.
5. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004. 350:2140–2150.
6. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000. 21:2071–2078.
7. Ratliff HL, Yousufuddin M, Lieving WR, Watson BE, Malas A, Rosencrance G, et al. Persistent left superior vena cava: case reports and clinical implications. Int J Cardiol. 2006. 113:242–246.
8. Jokinen JJ, Turpeinen AK, Pitkanen O, Hippelainen MJ, Hartikainen JE. Pacemaker therapy after tricuspid valve operations: implications on mortality, morbidity, and quality of life. Ann Thorac Surg. 2009. 87:1806–1814.
9. Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, et al. Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart. 2004. 90:307–313.
10. Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace. 2010. 12:103–108.
11. Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest. 2004. 126:1177–1186.
12. Burney K, Burchard F, Papouchado M, Wilde P. Cardiac pacing systems and implantable cardiac defibrillators (ICDs): a radiological perspective of equipment, anatomy and complications. Clin Radiol. 2004. 59:699–708.
13. Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J. 1995. 73:571–575.
14. Edwards NC, Varma M, Pitcher DW. Routine chest radiography after permanent pacemaker implantation: is it necessary? J Postgrad Med. 2005. 51:92–96. discussion 96-97.
15. Castillo R, Cavusoglu E. Twiddler's syndrome: an interesting cause of pacemaker failure. Cardiology. 2006. 105:119–121.
16. Magney JE, Flynn DM, Parsons JA, Staplin DH, Chin-Purcell MV, Milstein S, et al. Anatomical mechanisms explaining damage to pacemaker leads, defibrillator leads, and failure of central venous catheters adjacent to the sternoclavicular joint. Pacing Clin Electrophysiol. 1993. 16:445–457.
17. Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000. 101:1297–1302.
18. Roelke M, O'Nunain SS, Osswald S, Garan H, Harthorne JW, Ruskin JN. Subclavian crush syndrome complicating transvenous cardioverter defibrillator systems. Pacing Clin Electrophysiol. 1995. 18:973–979.
19. Mahapatra S, Bybee KA, Bunch TJ, Espinosa RE, Sinak LJ, McGoon MD, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm. 2005. 2:907–911.
20. Kiviniemi MS, Pirnes MA, Eranen HJ, Kettunen RV, Hartikainen JE. Complications related to permanent pacemaker therapy. Pacing Clin Electrophysiol. 1999. 22:711–720.
21. Ho WJ, Kuo CT, Lin KH. Right pneumothorax resulting from an endocardial screw-in atrial lead. Chest. 1999. 116:1133–1134.
22. Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol. 2007. 30:28–32.
23. Snow ME, Agatston AS, Kramer HC, Samet P. The postcardiotomy syndrome following transvenous pacemaker insertion. Pacing Clin Electrophysiol. 1987. 10:934–936.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download