Abstract
Objective
To evaluate the usefulness of percutaneous aspiration thromboembolectomy (PAT) via a transbrachial approach in patients with acute upper limb ischemia.
Materials and Methods
From July 2004 to March 2008, eleven patients with acute upper limb ischemia were enrolled in this study. They were initially treated with thrombolysis (n = 1), PAT (n = 6), or both (n = 4) via a femoral artery approach. However, all of the patients had residual thrombus in the brachial artery, which was subsequently managed by PAT via the transbrachial approach for removal of residual emboli.
Results
Successful re-canalization after PAT via a transbrachial approach was achieved in all patients. Two patients experienced early complications: one experienced a massive hematoma of the upper arm due to incomplete compression and was treated by stent deployment. The other patient experienced a re-occlusion of the brachial artery the day after the procedure due to excessive manual compression of the puncture site, but did not show recurrence of ischemic symptoms in the artery of the upper arm. Clinical success with complete resolution of ischemic symptoms was achieved in all patients.
Although acute upper limb ischemia is much less common than ischemia of the lower limbs, it can nevertheless result in severe functional impairment, even in the 601absence of overt tissue loss (1, 2). In 1962, Fogarty et al. (3) developed a balloon catheter for treatment of acute arterial occlusions. Use of this method resulted in dramatic simplification of the technical aspects of surgical therapy for acute arterial occlusion; however, the continued high incidence of perioperative and late morbidity and mortality associated with Fogarty balloon embolectomy and emergency reconstruction prompted a rethink of this approach (4, 5). Selective intra-arterial thrombolysis and mechanical thrombectomy have gained increasingly widespread acceptance as first-line therapies for acute lower limb ischemia (6-12). However, reports on its use in treatment of acute upper limb arterial occlusion have been limited (13, 14). The purpose of this study was to evaluate the usefulness of percutaneous aspiration thromboembolectomy (PAT) via a transbrachial approach in patients with acute upper limb ischemia.
Clinical records of all consecutive patients who underwent endovascular treatment for acute upper limb ischemia, from July 2004 to March 2008, were retrospectively evaluated. An Institutional Review Board exemption for the study was obtained. Two patients who presented following trauma were excluded from this study due to use of a surgical approach to the arterial rupture. During this period, 11 patients with acute upper limb ischemia, including seven men and four women, were enrolled in this study. The mean age was 74.5 years (range, 63-84 years). These patients had clinically acute limb ischemia, including coldness and pain in the upper extremity. The mean duration of acute limb ischemia was 2.2 days (range, 0-4 days). Prior to angiography, all patients underwent upper extremity CT for evaluation of the embolus location and collateral flow.
A transfemoral approach was used in all cases, with placement of a 7-Fr introducer (Radifocus; Terumo, Tokyo, Japan) into the common femoral artery. A subclavian artery angiography was performed in each patient using digital subtraction angiography (Artis dBA: Siemens, Erlangen, Germany) and arterial contrast injections (Visipaque 320; GE Healthcare, Princeton, NJ) with standard 5-Fr catheters (Radifocus; Terumo, Tokyo, Japan). After confirmation of the arterial obstruction of the upper extremity by angiography, endovascular treatments were initially performed as a thrombolysis or thrombus maceration using a balloon catheter through a transfemoral route, and PAT was performed via a transbrachial approach in patients with residual thrombi after endovascular treatment via a transfemoral approach.
All patients were treated with thrombolysis (n = 1), PAT (n = 6), or both (n = 4) via a transfemoral approach according to degree and duration of ischemic symptoms. We initially considered PAT via a transfemoral approach in patients with severe pain and coldness and thrombolysis in patients with mild pain and coldness. Thrombolytic infusion was performed using a 5-Fr multiple-side hole catheter system (Multi-Sideport Catheter Infusion Set; Cook, Bloomington, IN). The catheter was placed into the embolic arterial segment, with continuous administration of urokinase (Green Cross, Yongin, Korea) at 250,000-600,000 IU (1000-2000 IU/kg/h, 100,000 IU/h) during a period of 2-12 hours. Patients underwent systemic anticoagulation with a 5000-U bolus of intravenous heparin followed by continuous administration in order to maintain a partial thromboplastin time between 87 and 117 seconds, unless oral warfarin had already been administered (International Normalized Ratio [INR], 2.0-3.0). Thrombin time, partial thromboplastin time, hemoglobin, hematocrit, and platelet counts were measured every four hours. If a patient had gum bleeding, thrombolytic therapy was stopped. Progress of lysis was monitored by angiography after urokinase infusion. PAT was performed via a transfemoral approach using a 6-Fr or 7-Fr guiding catheter (Guider Softip; Boston Scientific, Natick, MA). After infusion of a thrombolytic agent or PAT via a transfemoral approach, selective angiography was performed for evaluation of the lesion. If there were any residual emboli in the axillary or upper brachial arteries, balloon dilatation was performed in order to achieve distal migration into the lower brachial artery for PAT via a transbrachial approach. Balloon dilatation was performed using a 6 mm × 4 cm catheter (OPTA; Cordis, Roden, The Netherlands).
Ultrasonography (US) of the upper arm was performed for determination of the best access route into the brachial artery for PAT. A transbrachial approach was used in all cases with placement of a 7-Fr introducer (Radifocus; Terumo, Tokyo, Japan) into the brachial artery. After selective angiography for evaluation of the extent of emboli, PAT was performed using a 6-Fr or 7-Fr guiding catheter (Guider Softip; Boston Scientific, Natick, MA).
Early complications were defined as procedure-related complications. Thrombolysis-related complications included puncture-site bleeding, guide-wire related arterial injury, and re-obstruction. Major complications included bleeding or hematoma necessitating transfusion and additional treatment, amputation, neurologic event, and death.
Patient follow-ups were conducted by clinic visits. For assessment of vessel patency, color Doppler US was performed at 1, 3, 6, and 12 months after the procedure and annually thereafter. Color Doppler US was evaluated from the subclavian artery to the ulnar and radial artery.
Initial technical success was defined as restoration of continuous inline flow through the brachial artery, and superficial and deep palmar arch. Clinical success was defined as the absence of symptoms related to arterial occlusion.
Table 1 summarizes all of the clinical results in this study.
Causes of acute upper limb ischemia, such as atrial fibrillation and congestive heart failure, were cardiac in origin in all patients. The lesion was right-sided in eight patients and left-sided in three. Three patients had an embolus in the axillary artery and nine had an embolus in the brachial artery.
Overall technical success of PAT via a transbrachial approach was 100%. On final angiography, three patients did not show any embolus in the brachial, ulnar, and radial arteries. Eight patients showed residual emboli in the radial or ulnar artery, but showed continuous flow into the branch of the radial or ulnar arch via a patent radial or ulnar artery (Fig. 1).
Early complications occurred in two patients. A massive hematoma due to swelling caused by bleeding from incomplete compression of the puncture site was detected in one patient (case 5) one day after the procedure. Color Doppler US showed continuous bleeding at the puncture site. Angiography via a transfemoral approach showed contrast extravasation at the puncture site, and balloon dilatation was performed for obliteration of the bleeding site. A self-expandable stent (8 mm × 6 cm, Wallstent; Boston Scientific, Natick, MA) was deployed at the bleeding site due to continuous bleeding after balloon dilatation. The final angiography showed no contrast extravasation at the puncture site (Fig. 2). This patient received anticoagulation therapy with warfarin sodium for six months. In another patient (case 6), re-occlusion of the brachial artery occurred due to extreme compression of the puncture site on color Doppler examination. However, this patient did not exhibit recurrence of ischemic symptoms in the artery of the upper arm, and was discharged without undergoing additional procedures. Therefore, clinical success consisted of complete resolution of symptoms in all patients.
The clinical and US follow-up period was 8-25 months (mean, 17. 2 months). One patient died of congestive heart failure two months after the procedure. On follow-up color Doppler US examination, no thrombus was observed in either the brachial or axillary artery. Seven patients with residual emboli in the radial or ulnar artery on final angiography, excluding one patient, who died of congestive heart failure, did not show residual emboli on color Doppler US after 1-3 months. No symptoms associated with arteries of the upper extremity were observed during the follow-up period. During the follow-up period, one patient experienced a stroke, and two exhibited lower leg ischemia due to migration of the embolus. Endovascular management for limb salvage was performed by removal of emboli on two patients with lower leg ischemia.
Numerous studies of acute arterial occlusion in the lower extremities have been conducted; however, little information is available regarding arterial occlusions of the upper extremities (12-16). An aggressive surgical approach has traditionally been advocated for management of acute upper limb ischemia (14-16). Surgically satisfactory results of acute upper limb ischemia were 91-100% (15, 16), with reported re-occlusion rates of 5-9%, an amputation rate of 2%, and a postoperative mortality rate of 6-9% after thromboembolectomy.
Endovascular management in patients with acute arterial occlusion included infusion of thrombolytic agents, such as urokinase and reteplase (6-9), and mechanical thrombectomy (10, 11). Ouriel et al. (6) reported that although intra-arterial infusion of urokinase in patients with acute arterial occlusion of the legs was associated with a higher frequency of hemorrhagic complications, intra-arterial infusion of urokinase and surgical treatment resulted in similarly significant improvement in the mean ankle brachial blood pressure index. Baguneid et al. (13) reported complete lysis of 50% of patients with acute upper limb ischemia by streptokinase. Five patients in our study who underwent urokinase infusion in the embolic arterial segment as the initial treatment did not show complete resolution of emboli. The infusion was performed using low-doses and a short time period of urokinase and for reduction of hemorrhagic risks by the relatively old-age of patients. In addition, we performed the mechanical procedure of PAT and angioplasty for achievement of rapid recovery from symptoms. Therefore, lysis of emboli by urokinase infusion was found to have an incomplete effect.
There have been few reports on PAT via a femoral artery approach in acute upper limb arterial occlusions. Due to the short length of the device and risk of embolic migration in the cerebral artery during the procedure, devices used for mechanical thromboembolectomy via a femoral artery approach are limited to use in patients with upper limb ischemia. Therefore, surgical thromboembolectomy in patients with acute upper limb ischemia is performed as the primary management (5, 15, 16).
In our series, we used the transbrachial approach for removal of emboli in patients with upper limb ischemia. PAT was performed using a 6-Fr or 7-Fr guiding catheter and continuous flow into the branches of the radial or ulnar arch was achieved via a patent radial or ulnar artery. Adequate performance of PAT can be achieved using a variety of generic, wide-lumen catheters, with no need for specific aspiration thromboembolectomy devices. Conventional thin-walled, big-lumen, guiding catheters, available in different shaft diameters, may be suitable for aspiration of thromboembolic material. The procedure can be repeated several times to ensure removal of the entire mass of thromboembolic material. The brachial approach to diagnostic angiogram and peripheral interventions results in outpatient examinations that are safer and more comfortable for the patient (17-21). Ernst et al. (21) reported that the transbrachial vascular approach for arterial interventions in iliac and femoro-popliteal pathologies is a reasonable alternative to transaxillary access if transfemoral puncture must be avoided. The technical success rate of arterial intervention is comparable with the results of transaxillary and transfemoral approaches. In our study, the clinical success of PAT via a transbrachial approach consisted of complete resolution in 91% of patients.
In our study, early complications after PAT via a transbrachial approach occurred in two patients, and these were associated with compression of the puncture site. Lienemann et al. (17) reported a 0.7% relevant local complication rate, without the need for surgical correction, in transbrachial angiography using 4-Fr and 5-Fr catheters. Chatziioannou et al. (20) reported a success rate in catheterization of one of the brachial arteries of 100%, with a low significant complication rate of 0.4%. Major complications associated with brachial artery catheterization included pseudoaneurysm, thrombosis, hematoma, or dissection. These complications after angiography via a transbrachial approach, excluding dissection, occurred due to incomplete compression of the puncture site. Endovascular management via a transbrachial approach was performed on cardiac and peripheral sections (21-24). Recently, satisfactory results for closure of the brachial artery puncture using Angioseal have been reported to reduce compression-related complications after the transbrachial approach at a rate of 97-100% (22, 25). Lupattelli et al. (22) reported a 3% rate of major complications, such as puncture site hematoma, occlusion, and pseudoaneurysm in diabetic patients after closure of brachial access using the Angioseal device during interventions for critical limb ischemia. The Angioseal is a valuable device for reduction of compression-related complications and can be used safely for transbrachial access in diabetic patients undergoing interventional procedures for critical limb ischemia.
There are some limitations to this study, mainly associated with a small sample size and a retrospective study design. In our hospital, surgical thromboembolectomy was performed on most patients with acute upper limb ischemia because it was the easiest route for removal of emboli and also due to the low risk of local anesthesia. In our study, the transbrachial approach for PAT in patients with acute upper limb ischemia achieved a high rate of technical and clinical success.
In conclusion, our results demonstrate that PAT via a transbrachial approach in patients with acute upper limb ischemia is a valuable, safe, and effective treatment. Use of this procedure will most likely minimize postoperative and anesthetic risks because most patients had cardiac problems associated with advanced age. Prospective and, preferably, multicenter studies are needed in order to further refine the technique and to validate the clinical usefulness of the procedures. In addition, we think that a comparison study of PAT with surgical thromboembolectomy as the initial treatment for acute upper limb ischemia should be conducted.
Figures and Tables
 | Fig. 173-year-old woman with acute embolus in right axillary artery (case 2).
A. Initial angiogram obtained in right subclavian artery showed embolus in axillary artery (arrows). B. Arteriogram after urokinase infusion and percutaneous aspiration thromboembolectomy via femoral artery showed residual embolus in upper brachial artery. Balloon dilatation was performed for distal migration of residual embolus into lower brachial artery (not shown). C. Arteriogram via transbrachial approach showed obstruction by distal emboli (arrow). D. Percutaneous aspiration thromboembolectomy was performed using 6-Fr guiding catheter for removal of emboli. E. Arteriogram after percutaneous aspiration thromboembolectomy showed continuous flow of radial artery and focal residual embolus in ulnar artery. There was no recurrence of ischemic symptoms in upper arm.
|
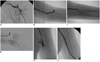 | Fig. 273-year-old woman with acute embolus in left axillary artery (case 5).
A. Initial angiogram obtained in left subclavian artery showed embolus in axillary artery (arrows). B. Arteriogram after urokinase infusion and percutaneous aspiration thromboembolectomy via femoral artery showed residual embolus in distal brachial artery (arrows). C, D. Arteriogram after percutaneous aspiration thromboembolectomy via transbrachial approach showed continuous flow of ulnar artery and arch and focal residual embolus (arrow) in ulnar artery. E. Arteriogram performed one-day after procedure due to massive hematoma and active bleeding at puncture site on color Doppler US showed contrast extravasation at upper brachial artery (arrows). F. Arteriogram performed after deployment of self-expandable stent at puncture site did not show any contrast extravasation.
|
References
1. Galbraith K, Collin J, Morris PJ, Wood RF. Recent experience with arterial embolism of the limbs in a vascular unit. Ann R Coll Surg Engl. 1985. 67:30–33.
2. Thompson JE, Sigler L, Raut PS, Austin DJ, Patman RD. Arterial embolectomy: a 20 year experience with 163 cases. Surgery. 1970. 67:212–220.
3. Fogarty TJ, Cranley JJ, Krause RJ, Strasser ES, Hafner CD. A method for extraction of arterial emboli and thrombi. Surg Gynecol Obstet. 1963. 116:241–244.
4. Thompson JE. Acute peripheral arterial occlusions. N Engl J Med. 1974. 290:950–952.
5. Schmidt FE, Hewitt RL. Severe upper limb ischemia. Arch Surg. 1980. 115:1188–1191.
6. Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998. 338:1105–1111.
7. Tepe G, Hopfenzitz C, Dietz K, Wiskirchen J, Heller S, Ouriel K, et al. Peripheral arteries: treatment with antibodies of platelet receptors and reteplase for thrombolysis--APART trial. Radiology. 2006. 239:892–900.
8. Valji K, Bookstein JJ, Roberts AC, Sanchez RB. Occluded peripheral arteries and bypass grafts: lytic stagnation as an end point for pulse-spray pharmacomechanical thrombolysis. Radiology. 1993. 188:389–394.
9. Muller-Hulsbeck S, Jahnke T. Peripheral arterial applications of percutaneous mechanical thrombectomy. Tech Vasc Interv Radiol. 2003. 6:22–34.
10. Ansel GM, Botti CF Jr, Silver MJ. Treatment of acute limb ischemia with a percutaneous mechanical thrombectomy-based endovascular approach: 5-year limb salvage and survival results from a single center series. Catheter Cardiovasc Interv. 2008. 72:325–330.
11. Ouriel K. Endovascular techniques in the treatment of acute limb ischemia: thrombolytic agents, trials, and percutaneous mechanical thrombectomy techniques. Semin Vasc Surg. 2003. 16:270–279.
12. Ricotta JJ, Scudder PA, McAndrew JA, De Weese JA, May AG. Management of acute ischemia of the upper extremity. Am J Surg. 1983. 145:661–666.
13. Baguneid M, Dodd D, Fulford P, Hadjilucas Y, Bukhari M, Griffiths G, et al. Management of acute nontraumatic upper limb ischemia. Angiology. 1999. 50:715–720.
14. Bergqvist D, Ericsson BF, Konrad P, Bergentz SE. Arterial surgery of the upper extremity. World J Surg. 1983. 7:786–791.
15. Hernandez-Richter T, Angele MK, Helmberger T, Jauch KW, Lauterjung L, Schildberg FW. Acute ischemia of the upper extremity: long-term results following thrombembolectomy with the Fogarty catheter. Langenbecks Arch Surg. 2001. 386:261–266.
16. Licht PB, Balezantis T, Wolff B, Baudier JF, Roder OC. Long-term outcome following thrombembolectomy in the upper extremity. Eur J Vasc Endovasc Surg. 2004. 28:508–512.
17. Lienemann A, Werle K, Weigert F. [Transbrachial arterial catheter angiography. Technique, indications, complications]. Radiologe. 1993. 33:102–107.
18. Watkinson AF, Hartnell GG. Complications of direct brachial artery puncture for arteriography: a comparison of techniques. Clin Radiol. 1991. 44:189–191.
19. Lederer W, Dingler WH, Gaa J, Brand H, Zoller W, Deininger HK. [The value of the transbrachial approach for arterial visualization using a 4-F catheter]. Rofo. 1989. 151:674–677.
20. Chatziioannou A, Ladopoulos C, Mourikis D, Katsenis K, Spanomihos G, Vlachos L. Complications of lower-extremity outpatient arteriography via low brachial artery. Cardiovasc Intervent Radiol. 2004. 27:31–34.
21. Ernst S, Fischbach R, Brochhagen HG, Heindel W, Landwehr P. Transbrachial thrombolysis, PTA and stenting in the lower extremities. Cardiovasc Intervent Radiol. 2003. 26:516–521.
22. Lupattelli T, Clerissi J, Clerici G, Minnella DP, Casini A, Losa S, et al. The efficacy and safety of closure of brachial access using the AngioSeal closure device: experience with 161 interventions in diabetic patients with critical limb ischemia. J Vasc Surg. 2008. 47:782–788.
23. Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J Am Coll Cardiol. 1997. 29:1269–1275.
24. Al-Mubarak N, Vitek JJ, Iyer SS, New G, Roubin GS. Carotid stenting with distal-balloon protection via the transbrachial approach. J Endovasc Ther. 2001. 8:571–575.
25. Belenky A, Aranovich D, Greif F, Bachar G, Bartal G, Atar E. Use of a collagen-based device for closure of low brachial artery punctures. Cardiovasc Intervent Radiol. 2007. 30:273–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download