1. Stehbens WE. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol. 1963; 75:45–64. PMID:
14087271.
2. Stehbens WE. Pathology of the cerebral blood vessels. 1972. St. Louis: C.V. Mosby.
3. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998; 29:251–256. PMID:
9445359.
4. Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958; 17:409–423. PMID:
13564252.
5. Chason JL, Hindman WM. Berry aneurysms of the circle of Willis; results of a planned autopsy study. Neurology. 1958; 8:41–44. PMID:
13493689.

6. Iwamoto H, Kiyohara Y, Fujishima M, Kato I, Nakayama K, Sueishi K, et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period. The Hisayama study. Stroke. 1999; 30:1390–1395. PMID:
10390312.
7. Rocque BG, Baskaya MK, Kuo JS. Incidental findings on brain MRI. N Engl J Med. 2008; 358:853. author reply 854-855. PMID:
18287610.

8. Horikoshi T, Akiyama I, Yamagata Z, Nukui H. Retrospective analysis of the prevalence of asymptomatic cerebral aneurysm in 4518 patients undergoing magnetic resonance angiography--when does cerebral aneurysm develop? Neurol Med Chir (Tokyo). 2002; 42:105–112. discussion 113. PMID:
11936051.
9. Ross JS, Masaryk TJ, Modic MT, Ruggieri PM, Haacke EM, Selman WR. Intracranial aneurysms: evaluation by MR angiography. AJNR Am J Neuroradiol. 1990; 11:449–455. PMID:
2112306.

10. White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology. 2000; 217:361–370. PMID:
11058629.

11. Chung TS, Joo JY, Lee SK, Chien D, Laub G. Evaluation of cerebral aneurysms with high-resolution MR angiography using a section-interpolation technique: correlation with digital subtraction angiography. AJNR Am J Neuroradiol. 1999; 20:229–235. PMID:
10094343.
12. Grandin CB, Mathurin P, Duprez T, Stroobandt G, Hammer F, Goffette P, et al. Diagnosis of intracranial aneurysms: accuracy of MR angiography at 0.5 T. AJNR Am J Neuroradiol. 1998; 19:245–252. PMID:
9504473.
13. Yue NC, Longstreth WT Jr, Elster AD, Jungreis CA, O'Leary DH, Poirier VC. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the Cardiovascular Health Study. Radiology. 1997; 202:41–46. PMID:
8988190.

14. Mallouhi A, Felber S, Chemelli A, Dessl A, Auer A, Schocke M, et al. Detection and characterization of intracranial aneurysms with MR angiography: comparison of volume-rendering and maximum-intensity-projection algorithms. AJR Am J Roentgenol. 2003; 180:55–64. PMID:
12490476.

15. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007; 357:1821–1828. PMID:
17978290.

16. Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990; 34:361–365. PMID:
2244298.

17. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362:103–110. PMID:
12867109.

18. Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991; 75:8–14. PMID:
2045924.
19. Russell SM, Lin K, Hahn SA, Jafar JJ. Smaller cerebral aneurysms producing more extensive subarachnoid hemorrhage following rupture: a radiological investigation and discussion of theoretical determinants. J Neurosurg. 2003; 99:248–253. PMID:
12924696.

20. Hwang JH, Roh HG, Chun YI, Kang HS, Choi JW, Moon WJ, et al. Endovascular coil embolization of very small intracranial aneurysms. Neuroradiology. 2011; 53:349–357. PMID:
20574735.

21. van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. 2009; 30:835–839. PMID:
19131407.
22. Gupta V, Chugh M, Jha AN, Walia BS, Vaishya S. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol. 2009; 30:308–314. PMID:
19001535.

23. Inagawa T, Hada H, Katoh Y. Unruptured intracranial aneurysms in elderly patients. Surg Neurol. 1992; 38:364–370. PMID:
1485213.

24. Chae KS, Jeon P, Kim KH, Kim ST, Kim HJ, Byun HS. Endovascular coil embolization of very small intracranial aneurysms. Korean J Radiol. 2010; 11:536–541. PMID:
20808697.

25. Okahara M, Kiyosue H, Yamashita M, Nagatomi H, Hata H, Saginoya T, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke. 2002; 33:1803–1808. PMID:
12105357.
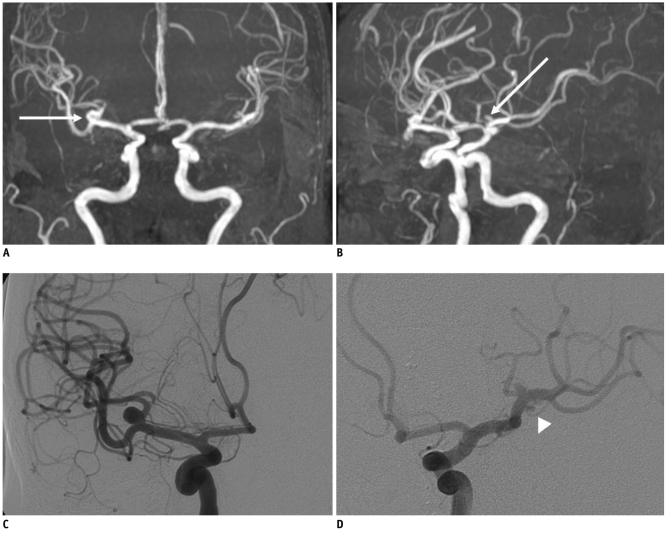
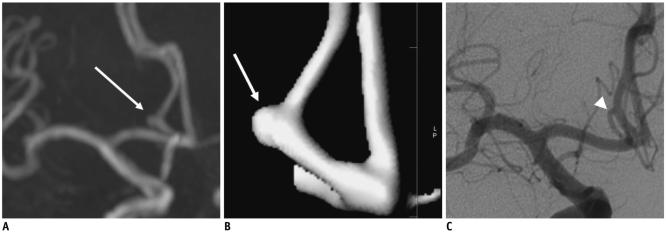




 PDF
PDF ePub
ePub Citation
Citation Print
Print


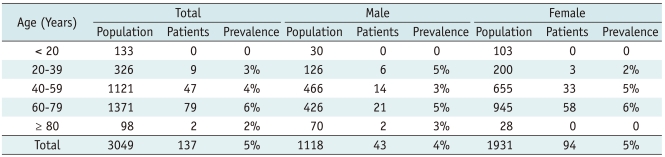
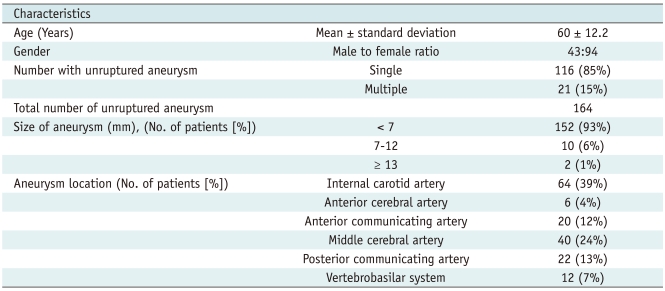
 XML Download
XML Download