Abstract
We report here on a case of localized lymphoid hyperplasia of the pancreas in a 70-year-old man which manifested as double lesions (uncinate process and tail) in the organ. The lesions were incidentally detected as hypoechoic lesions on ultrasonography and they appeared as delayed enhancing lesions on the contrast-enhanced dynamic CT and MRI. Total pancreatectomy was performed, because malignant tumor could not be excluded according to the preoperative imaging studies and the endoscopic ultrasound-guided biopsy failed. Pathology revealed localized lymphoid hyperplasia. The patient had an uneventful postoperative course. He has been alive for 18 months after surgery.
Localized lymphoid hyperplasia is also called "pseudolymphoma", and this has been found in various organs, including the skin, orbit, thyroid, breast, lung, gastrointestinal tract, liver and pancreas (1-6). It is characterized by the presence of hyperplastic lymphoid follicles with populations of polymorphic and polyclonal cells, and these cell populations are composed of small mature lymphocytes, mature plasma cells, macrophages and stromal fibrosis.
Localized lymphoid hyperplasia of the pancreas is extremely rare and it has rarely been described in the literature (1-3). The imaging findings of pancreatic localized lymphoid hyperplasia are not well known. Thus, it is still indistinguishable from other pancreatic neoplasms both clinically or radiographically. To the best of our knowledge, only three cases of pancreatic localized lymphoid hyperplasia have been reported in the English medical literature (1-3). Moreover, these reports included solitary localized lymphoid hyperplasia of the pancreas and the imaging findings were briefly described as a hypoechoic lesion on ultrasonography (US) with a normal arterial distribution on angiography (2). We report here on a case of double localized lymphoid hyperplasia of the pancreas with an emphasis on the imaging findings of ultrasonography, computed tomography (CT) and magnetic resonance (MR) imaging.
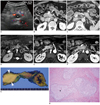
A 70-year-old man was admitted to our hospital for the evaluation of a pancreatic mass that was incidentally detected during a screening abdominal sonographic examination. He was asymptomatic and he did not have any specific medical history. He had a habit of drinking one or two cups of alcohol daily for several decades. The laboratory tests at the time of admission revealed that the CA19-9, CEA, alpha-fetoprotein, amylase and lipase levels were within the normal limits. The ultrasonographic images showed a hypoechoic mass in the uncinate process of the pancreas, and this was not accompanied by dilatation of the main pancreatic duct. There was no discernible vascularity within the mass on the color Doppler image (Fig. 1A). We then performed contrast-enhanced multidetector-row CT. The contrast-enhanced dynamic CT images revealed two round masses with one each in the uncinate process (about 2 × 1.8 cm for the mass size) and tail (about 1.8 × 1.5 cm for the mass size) of the pancreas; these isodense masses on the unenhanced CT images were slightly hypodense, slightly hyperdense and slightly hyperdense, respectively as compared to the pancreas parenchyma on the contrast-enhanced arterial, portal, and delayed-phase CT images that were obtained at 30 seconds, 75 seconds and 180 seconds, respectively, after the injection of contrast agent (Fig. 1B, C). There were no enlarged lymph nodes in the abdomen. MR imaging was performed to further evaluate these pancreatic masses. The MR images demonstrated that the two masses were hypointense and hyperintense on the T1-and T2-weighted images, respectively (Fig. 1D). Although subtle enhancement of the two masses was detected, these lesions were shown to be of slight hypointensity as compared to the intensity of the pancreas parenchyma on the gadolinium-enhanced arterial phase T1-weighted image obtained at 25 seconds after the injection of contrast agent (Fig. 1E). Meanwhile, the mass lesions were more enhanced than the pancreas parenchyma on the portal and delayed-phase T1-weighted MR images (Fig. 1F) obtained at 60 seconds and 180 seconds, respectively, after the injection of contrast agent.
Several imaging findings of these tumors, such as the delayed enhancement, the double lesions and the normal caliber of the main pancreatic duct, resulted in the preoperative tentative diagnosis of neuroendocrine tumor or lymphoma. Under this impression, endoscopic US-guided biopsy was performed, but this failed. Finally, total pancreaticoduodenectomy was performed for these masses because the malignant potential of these masses could not be ruled out.
The gross surgical specimen showed two well circumscribed yellowish masses in the uncinate process and tail of the pancreas (Fig. 1G). Microscopic examination revealed lymphoid follicles with enlarged germinal centers and dense, rich collagenous fibrous tissue admixed with a lymphoplasma cell infiltration (Fig. 1H). The immunohistochemical stains demonstrated that the follicular lymphocytic infiltrate of a polyclonal origin was composed of germinal centers with cells expressing B-cell characteristics and these germinal centers were surrounded by rims of cells expressing T-cell characteristics. No immunoreactivity for IgG4 was noted. These histologic features resulted in the diagnosis of localized lymphoid hyperplasia of the pancreas. He had an uneventful postoperative course and he has been alive for 18 months after surgery.
Localized lymphoid hyperplasia can manifest as a focal mass-like lesion that is well-defined from the surrounding tissue (2). Localized lymphoid hyperplasia has been described as a hypoechoic mass on ultrasonograms and as a well demarcated mass on CT images (2, 4, 5). In our case, the localized lymphoid hyperplasia was visualized in the form of a discernible mass with delayed enhancement on the imaging studies. Given that the border between the localized lymphoid hyperplasia and the surrounding pancreas parenchyma was sharply demarcated on the pathologic specimen, it seems to be natural that the localized lymphoid hyperplasia was seen as a circumscribed round mass on all imaging studies. In the previous reports (4, 5) on lymphoid hyperplasia of the liver, the lesions appeared to be hypo- and hyperintense nodules on the T1- and T2-weighted images, respectively, which is similar to our case. In regard to contrast enhancement, reactive lymphoid hyperplasia of the liver showed a variety of dynamic enhancement patterns from arterial hypervascularity followed by washout to arterial hypovascularity with slight enhancement. In our case, the localized lymphoid hyperplasia of the pancreas showed arterial hypovascularity with delayed enhancement. We think that the enhancement pattern in our case was attributed to the presence of a rich fibrous matrix in the lesions.
The dilatation of the pancreatic duct is one of valuable additional findings for characterizing any pancreatic tumor. The presence of dilatation of the pancreatic duct that is induced by a pancreatic mass usually increases the possibility of malignant tumor. According to the previous reports (2, 3), pancreatic localized lymphoid hyperplasia was accompanied with the dilatation of the pancreatic duct or common bile duct, and this was interpreted to be the result of the mass effect of localized lymphoid hyperplasia on the pancreatic duct or the common bile duct. In our case, the diameter of the pancreatic duct showed a normal caliber. This may reflect that pancreatic localized lymphoid hyperplasia is unrelated to the pancreatic duct for its origin and the tumor has a benign histology. In addition, the one mass that was located in the uncinate process seemed to have no mass effect or a minimal mass effect on the main pancreatic duct.
Localized lymphoid hyperplasia is not considered to have any malignant potential (4, 5). Given that pseudolymphoma may denote a low-grade mucosa-associated lymphoid tissue (MALT) lymphoma, and malignant transformation of pseudolymphoma has been sporadically reported in the literature (5, 7), the term "localized lymphoid hyperplasia" seems to more accurately reflect the pathologic findings of our case than the term "pseudolymphoma".
The differential diagnosis of localized lymphoid hyperplasia of the pancreas includes neuroendocrine tumor, autoimmune pancreatitis, lymphoma and solid pseudopapillary tumor. Although neuroendocrine tumors are usually thought to be seen as hypervascular masses on the arterial phase images, the degree, uniformity and temporal change of the enhancement on the dynamic contrast-enhanced images can be highly variable (8). Especially, more attention needs to be paid to distinguish between a delayed-enhancing, nonfunctioning neuroendocrine tumor and localized lymphoid hyperplasia. Given the imaging findings of localized lymphoid hyperplasia of the pancreas in this case, the presence of cystic or necrotic change in the mass may favor a diagnosis of neuroendocrine tumor. The localized form of autoimmune pancreatitis may present with a focal mass-like lesion and it share several imaging features with our case, such as delayed enhancement, a nondilated pancreatic duct and tumor multiplicity (9). However, the localized form of autoimmune pancreatitis was reported to be often accompanied by wall thickening and enhancement of the common bile duct and upstream dilatation, and particularly in the cases whose pancreatic head was involved (9), which may be diagnostic points to differentiate it from localized lymphoid hyperplasia. Pancreatic lymphoma sometimes manifests as a localized, well circumscribed mass in the pancreas, and this is not accompanied by dilatation of the pancreatic duct (10). In contrast with pancreatic localized lymphoid hyperplasia, pancreatic lymphoma appears as a heterogeneous mass with low to intermediate signal intensity on the T2-weighted images and as a hypovascular mass on the contrast-enhanced images. Finally, solid pseudopapillary tumor (SPT) needs to be included in the list of the differential diagnoses because small (less than 3 cm) SPTs can appear as well-defined solid lesions with delayed enhancement (11).
In summary, we present here an interesting case of double pancreatic localized lymphoid hyperplasia with the US, CT and MR imaging findings. Although it is rare, localized lymphoid hyperplasia of the pancreas should be included in the differential diagnosis of pancreatic tumor, and especially when the imaging studies show a localized, delayed enhancing mass of the pancreas without any dilatation of the pancreatic duct.
Figures and Tables
Fig. 1
Localized lymphoid hyperplasia of pancreas in 70-year-old man.
A. Color Doppler ultrasonographic image shows hypoechoic mass (asterisk) in uncinate process of pancreas without discernible vascularity within mass. A = aorta, S = superior mesenteric vein. B, C. Contrast-enhanced 3-minute delayed phase CT images reveal two localized masses in uncinate process (about 2 × 1.8 cm for mass size) and tail (about 1.8 × 1.5 cm for mass size) of pancreas without peripancreatic stranding (arrows in B and arrowheads in C), and these masses show slightly more enhancement, as compared to that of pancreatic parenchyma. D. T2-weighted MR image shows localized hyperintense mass without peripancreatic stranding in uncinate process of pancreas (arrow). E, F. Gadolinium-enhanced dynamic T1-weighted images show slightly hypointense mass (arrow in E) on arterial phase image (E) in uncinate process of pancreas, and mass appears as slightly hyperintense on portal phase image (F). G. Photograph of resected specimen shows well circumscribed yellowish mass (arrowheads) in uncinate process of pancreas. H. Photomicrograph shows lymphoid follicles (asterisk) surrounded by dense collagenous tissue. Lymphoid follicles are composed of enlarged germinal centers surrounded by rims of small lymphocytes (Hematoxylin & Eosin staining, × 100). On immunohistochemical staining, neither lymphocytic infiltrate with monoclonal cell proliferation nor immunoreactivity for IgG4 was found in lesions.
References
1. Babaryka I, Thomas E. [Pancreatitis lymphomatosa (author's transl)]. Zentralbl Allg Pathol. 1981. 125:315–318.
2. Hatzitheoklitos E, Buchler MW, Friess H, DiSebastiano P, Poch B, Beger HG, et al. Pseudolymphoma of the pancreas mimicking cancer. Pancreas. 1994. 9:668–670.
3. Nakashiro H, Tokunaga O, Watanabe T, Ishibashi K, Kuwaki T. Localized lymphoid hyperplasia (pseudolymphoma) of the pancreas presenting with obstructive jaundice. Hum Pathol. 1991. 22:724–726.
4. Machida T, Takahashi T, Itoh T, Hirayama M, Morita T, Horita S. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol. 2007. 13:5403–5407.
5. Takahashi H, Sawai H, Matsuo Y, Funahashi H, Satoh M, Okada Y, et al. Reactive lymphoid hyperplasia of the liver in a patient with colon cancer: report of two cases. BMC Gastroenterol. 2006. 6:25.
6. Tokunaga O, Morimatsu M, Nakashima T. Pseudolymphoma of the stomach. Nodular type. Acta Pathol Jpn. 1985. 35:227–231.
7. Schwartz MS, Sherman H, Smith T, Janis R. Gastric pseudolymphoma and its relationship to malignant gastric lymphoma. Am J Gastroenterol. 1989. 84:1555–1559.
8. Herwick S, Miller FH, Keppke AL. MRI of islet cell tumors of the pancreas. AJR Am J Roentgenol. 2006. 187:W472–W480.
9. Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics. 2008. 28:157–170.
10. Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000. 174:671–675.
11. Baek JH, Lee JM, Kim SH, Kim SJ, Lee JY, Han JK, et al. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010. 257:97–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download