Abstract
Rectal submucosal lesions encompass a wide variety of benign and malignant tumors involving the rectum. With optical colonoscopy, any mass-like protrusion covered by normal mucosa, whether the underlying process is intramural or extramural in origin, may be reported as a submucosal lesion. Whereas the assessment of submucosal lesions may be limited with performing optical colonoscopy, cross-sectional imaging such as CT, transrectal ultrasonography and MRI allows the evaluation of perirectal tissues and pelvic organs in addition to the entire thickness of the rectum, and so this is advantageous for the assessment of rectal submucosal tumors. Among these, MRI is the best investigative modality for soft tissue characterization. Therefore, knowledge of the MRI features of rectal submucosal tumors can help achieve accurate preoperative diagnoses and facilitate the appropriate management.
Rectal submucosal lesions encompass a wide variety of benign and malignant tumors that involve the rectum. A common pathological feature of submucosal lesions is that they arise in the deep layers of the rectal wall (the submucosa or muscularis propria) with normal overlying mucosa. With performing optical colonoscopy, the term "submucosal lesions" may be used to describe any mass-like protrusion into the lumen that spares the overlying mucosa, without regard to an intramural versus extramural origin (1). Therefore, to arrive at a reasonable differential diagnosis of rectal submucosal lesions, disease processes that give rise to both intramural and extramural lesions should be considered (1, 2) (Table 1).
Although optical colonoscopy provides excellent visualization of the luminal aspect of mucosal lesions and it enables endoscopic biopsy, it has limitations to evaluate the full-thickness of these submucosal lesions. In contrast, cross-sectional imaging allows evaluating perirectal tissues and pelvic organs in addition to the entire bowel thickness. So, the cross-sectional imaging modalities such as computed tomography (CT), transrectal ultrasonography and magnetic resonance imaging (MRI) may help characterize rectal submucosal lesions as the next step following their detection by optical colonoscopy.
In particular, MRI has advantages in characterizing soft tissues because of its higher soft-tissue contrast to noise ratio, and MRI demonstrates the layers of the rectal wall with higher resolution. The multiplanar and three-dimensional images from MRI also enable clarification of the relationship between rectal submucosal lesions and the pelvic floor or the bony pelvis. Therefore, the purpose of this review is to illustrate the characteristic MRI features of rectal submucosal lesions as classified by an intramural or extramural origin.
Leiomyomas of the rectum are rare, benign smooth muscle tumors that are uniformly positive for smooth muscle actin and desmin, and they are not kit-positive in contrast to that of gastrointestinal stromal tumors (GISTs). In the rectum, leiomyomas are encountered more frequently than lipomas. They originate from either the muscularis mucosa or muscularis propria and they are usually located in the distal rectum. Most leiomyomas arising from the muscularis mucosa appear as small, sessile, asymptomatic polyps. Leiomyomas from the muscularis propria may show a larger size and symptoms such as bleeding, discomfort or stenosis. The MRI findings of rectal leiomyomas have not been well described, but it is reported that they are isointense to mildly hyperintense on T2-weighted images (T2WI) when compared to that of muscle (3) (Fig. 1).
Lipoma is a rare, benign, slow-growing mesenchymal tumor. It is the second most common benign tumor in the rectum after leiomyomas, whereas it has been reported to be the most frequently encountered submucosal tumor in the colon. Rectal lipomas may cause rectal bleeding, pain or tenesmus. Liposarcomatous degeneration has not been reported and if the patient remains asymptomatic, then treatment is usually not necessary (1).
On imaging studies, most lipomas present as a broad-based mass with a smooth, well-demarcated margin. Although submucosal in origin, lipomas occasionally evolve into pedunculated lesions (1). MRI readily demonstrates the fatty nature of the mass as being isointense compared to the subcutaneous fat on T1WIs, and low signal intensity on T1WI with fat suppression (Fig. 2), which confirms the diagnosis of lipoma.
Based on the WHO classification of 2000, neuroendocrine tumors are categorized into three groups; well-differentiated neuroendocrine tumor (NET) (benign behavior or uncertain malignant potential; synonymous to carcinoids in gastroenteric NETs), well-differentiated neuroendocrine carcinoma (low-grade malignancy; synonymous to malignant carcinoid) and poorly-differentiated neuroendocrine carcinomas (high-grade malignancy; usually small cell neuroendocrine carcinomas) (4).
Rectal NETs are much more frequently observed than colon NETs, and the former usually shows a lower risk of metastasis and so they have a better prognosis. Tumor size and microinvasiveness are probably the two most important prognostic factors upon which treatment decisions may be based (< 1 cm: endoscopic or trans-anal resection; 1-2 cm without muscular invasion or lymph node metastasis: wide excision; 2 cm or greater, muscular invasion or lymph node metastasis: radical surgery). Metastasis are only likely in tumors that are 2 cm and larger and they invade the muscularis mucosa (4, 5).
Typically, rectal carcinoid is seen on MRI as a single discrete superficial submucosal mass smaller than 1 cm with contrast enhancement. Carcinoid tumors develop from the muscularis mucosa or the submucosal layer (6), so a superficial location or an intraluminal growing pattern may be related with the superficial layer of origin. The signal intensity of the lesion is usually isointense on T1WI and isointense to hyperintense on T2WI in comparison to muscles, and carcinoid tumors are homogeneously enhanced by contrast injection (3) (Fig. 3). Most poorly-differentiated neuroendocrine carcinomas are small cell carcinomas, and these are high-grade malignancy. Early dissemination and rapid clinical deterioration are common characteristics of this disease entity, which is one of the most aggressive tumors arising at the rectum (7). The usual imaging finding is similar to that of adenocarcinoma; an ulcerative tumor with or without metastases (8) (Fig. 4). If a lesion is discovered to be a neuroendocrine carcinoma, then it should be treated as an adenocarcinoma (9).
Gastrointestinal stromal tumors harbor kit mutations that cause the overexpression of kit protein (CD 117) and these tumor show differentiation toward (or they are derive from) interstitial cells of Cajal. The malignant potential (very low, low, intermediate and high risk) depends on the mitotic rate and the size of the tumor (10) (Fig. 5). The sites most commonly involved by GIST are the stomach (60-70%) and small intestine (20-25%), with about 5% of all GISTs arising at the rectum (11). It is generally agreed that complete surgical resection with negative tumor margins is the principal curative procedure for primary, non-metastatic tumors.
Gastrointestinal stromal tumors are usually seen as an eccentric mural mass with welldefined tumor margins. Calcification, necrosis and ulceration may be observed in the intermediate and high-risk tumors (12) (Fig. 6). The MRI features described for rectal GISTs are well-defined masses with low signal intensity on T1WI, they are hyperintense or isointense with hyperintense areas on T2WI and they exhibit marked heterogeneous enhancement after gadolinium administration (12).
Schwannomas are uncommon stromal tumors that are considered to arise from neuron-supporting, site-specific, modified Schwann cells in the mesenteric plexus, and they may develop in any anatomic compartment. Nerve fibers stretch around the mass and the tumor is usually encapsulated (13).
Among the case reports of schwannoma in the digestive tract, the most frequent sites are the esophagus and stomach, followed by the small intestine and the appendix. Schwannoma of the rectum is extremely rare (13, 14) (Fig. 7). The imaging diagnosis is often confusing, and differentiating them from other rectal submucosal tumors such as leiomyomas or GISTs is difficult even based on conventional pathological techniques. Immunohistochemical studies should be done for making a more accurate diagnosis.
Primary rectal lymphoma involving the rectum is a rare type of gastrointestinal lymphoma. The diagnosis of primary colorectal lymphomas requires that there is a lymphoproliferative disorder confined to the colorectum and regional lymph nodes without involvement in the abdominal organs and bone marrow, and neither retroperitoneal nor medial lymphadenopathy is present (15).
Gastrointestinal lymphoma has usual features such as a bulky mass or diffuse infiltration. Preservation of the extramural fat planes is more likely to be seen in lymphoma than in adenocarcinoma in the presence of a bulky tumor. In addition, luminal obstruction is rare even when there is extensive lymphomatous infiltration. However, gastrointestinal tract lymphoma may have diverse radiologic manifestations and it may mimic adenocarcinoma. The MRI features of lymphoma usually show homogeneous intermediate signal intensity on T1WI and high signal intensity on T2WI (Fig. 8). Mild to moderate enhancement is seen after the intravenous administration of gadolinium-based contrast material (16). Treatment often involves a multimodality approach that combines surgery and chemotherapy, and radiotherapy is used in selected cases (17).
Malignant melanoma is a highly malignant tumor that rarely arises at the anorectum. It has been reported that anorectal malignant melanoma makes up 2.3% of all non-cutaneous-origin melanomas and 0.1% of all rectal malignancies. Since melanosis is often found in non-cutaneous melanoma patients, the origin of anorectal malignant melanoma might be the melanocytes in the squamous epithelium of the anal canal (18).
Malignant melanoma usually presents as a bulky intraluminal fungating mass in the distal rectum. It focally expands and obscures the lumen without causing obstruction and there is perirectal infiltration and frequently enlarged lymph nodes (19) (Fig. 9). Melanoma cells carry melanin pigments that contain free radicals, and this makes the cells paramagnetic, which consequently shortens the T1 relaxation time and increases the T2 relaxation time on MRI. This accounts for the high-in-low intensity seen on T1WI and the mixed intensity seen on T2WI (18, 20). Based on these MRI features, a few reports have stated that MRI is useful for the characterization of malignant melanoma (20). However, approximately 10% to 29% of anorectal melanomas are known to be the amelanotic type, and the MRI findings resemble rectal cancer (18, 20). Sometimes metastatic lymph nodes might contain pooled hemorrhage, and this presents as a high-intensity signal on MRI (20).
Endometriosis is defined as the presence of functioning endometrial glands and stroma outside the uterine cavity. This entity should be considered in the differential diagnosis if an extramucosal rectal mass is seen in premenopausal women.
The typical MRI features are high signal intensity on both T1WI and T2WI, and persisting high signal intensity is observed on fat suppressed T1WI (Fig. 10). T2WI can display a gradual variation of signal intensity and this phenomenon is described as "shading," and it is due to chronic bleeding with accumulated iron and protein. However, the disease can also present as either fibrous masses that appear to show speculation and retraction and hypointense signals on both T1WI and T2WI or fibrous masses with punctuate foci of hyperintense signals on T1WI and T2WI (21).
Developmental disorders should be considered in the differential diagnosis of retrorectal masses that have major cystic components. These lesions may have similar imaging features. Rough differentiation is possible by the fact that epidermoid cyst, dermoid cyst and rectal cystic duplication are more often unilocular, whereas the tailgut cysts tend to be multilocular. Demonstration of fat components is suggestive of a dermoid cyst. Rectal duplications often have communications with the rectal lumen and they are anterior to the rectum (22). However, the role of MRI for the detection of calcification is limited, and this should be supplemented with evaluation by other modalities, and especially when assessment of calcification is crucial for narrowing the possibility of dermoid cyst or teratoma.
Tailgut cyst is a rare developmental lesion that occurs in the retrorectal space. It presents as a multicystic or unilocular mass that usually adheres to the sacrum or rectum, but it does not communicate with the rectal lumen. Malignant degeneration into mucinous adenocarcinoma is a rare complication, but dysplastic epithelial change is common.
Tailgut cyst usually occurs as a multicystic lesion, but unilocular tailgut cysts have occasionally been reported. The presence of small cysts clustered together adjacent to a main cyst producing a honeycomb pattern is frequently observed (22). MRI enables the detection of mucin and blood in a cystic lesion, but the signal intensity of mucinous fluid is variable and depends on the protein concentration. T1WI may show a signal intensity change from hypointense to hyperintense as the protein concentration increases (Fig. 11). On T2WI, the signal intensity of mucinous fluid could decrease from highly hyperintense to hypointense as the protein concentration and viscosity increase. The potential complications of tailgut cyst include infection with recurrent fistula and on rare occasions malignant transformation (22) (Fig. 12).
Alimentary tract duplications are uncommon congenital malformations. They can be associated with a fistula to the anus or the peri-anal region. The criteria for defining alimentary tract duplication include 1) contiguity and adherence to some part of the alimentary tract, 2) a smooth muscle coat and a mucosal lining consisting of one or more types of cells found within the alimentary tract. On cross-sectional imaging duplication cysts generally have a largely exoenteric location (2) (Fig. 13). The most common morphological feature is a spherical or sausage-shaped cystic structure with a muscular wall of varying thickness and completeness. The cyst wall often has its own mucosa and submucosa, and the cyst wall may show some splitting of the muscle coats. The presence of a muscle coat enables reliable differentiation of a duplication cyst from a tailgut cyst as a muscle coat is absent in the latter.
Epidermoid cyst is a rare congenital disease that develops from the remnants of ectodermal tissues misplaced during embryogenesis. Histologically it consists of a thin wall lined by stratified squamous epithelium and this surrounds a mixture of desquamated debris, cholesterol, keratin and water, but it lacks the skin appendages such as sweat glands, hair follicles and sebaceous glands, which are seen in dermoid cysts (23). On MRI, a mass exhibiting heterogeneous low signal intensity on T1WI and high signal intensity on T2WI, and the mass contains multiple small hypointense signal intensity debris that represent aggregates of keratin, helps to differentiate this lesion from other presacral cystic lesions (23) (Fig. 14).
Rectal involvement may occur via contiguous tumor spread from adjacent organs such as ovary or prostate (Fig. 15). Direct invasion of the rectum by an extra-rectal neoplasm may sometimes mimic rectal intramural submucosal tumors, and even on MRI and optical colonoscopy.
A variety of tumors and tumor-mimicking mass lesions can involve the rectal submucosal or the perirectal compartment. These include primary benign or malignant tumors, metastatic tumors and developmental lesions. MRI can play an essential role in the preoperative detection and characterization of masses, and MRI can yield useful information for the differential diagnosis and for establishing treatment strategies.
Figures and Tables
 | Fig. 1Rectal leiomyoma in 42-year-old woman.
Axial T2-weighted image (A), T1-weighted image (B) and gadolinium enhanced axial T1-weighted image (C) demonstrated submucosal mass (arrows) in anterior wall of distal rectum. Mass is well-demarcated and it shows signal intensity that is slightly brighter than that of skeletal muscles on T2-weighted image.
|
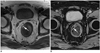 | Fig. 2Rectal lipoma in 74-year-old man.
Axial T1-weighted image (A) demonstrates oval shaped rectal submucosal mass (arrow) with signal density identical to that of perirectal fat tissue. On gadolinium enhanced axial T1-weighted image (B) signal of lesion drops due to fat suppression, which confirms diagnosis of lipoma (arrow).
|
 | Fig. 3Rectal carcinoid (well-differentiated neuroendocrine tumor) in 60-year-old man.
Axial T2-weighted image (A), axial T1-weighted image (B) and gadolinium enhanced axial T1-weighted image with fat suppression (C) show discrete 2.5-cm homogeneously enhancing intraluminal submucosal mass in rectum (arrows).
|
 | Fig. 4Poorly-differentiated neuroendocrine carcinoma in 61-year-old woman which was diagnosed as small cell carcinoma.
Colonoscopy (A) shows ulcerofungating mass (white arrows) in lower rectum, which is similar to adenocarcinoma. Axial T2-weighted image (B) and gadolinium enhanced coronal T1-weighted image (C) also demonstrate fungating mass (white arrows) in lower rectum. Enlarged metastatic lymph node (arrowheads) is observed in mesorectum.
|
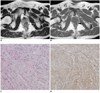 | Fig. 5Rectal gastrointestinal stromal tumor in 59-year-old woman.
Axial T2-weighted image (A) and T1-weighted image (B) show 1.5-cm submucosal mass (arrows) in left anterior lateral wall of lower rectum. Mass is relatively well-demarcated. After ultralow anterior resection, histopathologic diagnosis was very low-risk gastrointestinal stromal tumor (mitotic rate: 0 per 50 high power fields) based on Hematoxylin & Eosin staining (× 200) (C) and positive c-kit results on immunohistochemistry (D).
|
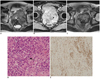 | Fig. 6Rectal gastrointestinal stromal tumor in 53-year-old man.
Axial T2-weighted image (A) and gadolinium enhanced axial T1-weighted image with fat suppression (B) revealed 9.6-cm multilobulated mass that was possibly arising from anterior wall of rectum. Within mass, dark signal intensities (arrows) due to internal calcification and fluid intensity (arrowheads) due to necrosis are seen. After imatinib treatment, prominent tumor shrinkage is demonstrated on follow-up axial T2-weighted image (C). Mile's operation was performed. Hematoxylin & Eosin staining (× 200) (D) and positive results of c-kit immunohistochemistry (E) revealed diagnosis of high-risk gastrointestinal stromal tumor, in which frequent mitosis (arrow in D; mitotic rate: more than 10 per 50 high power fields) was observed.
|
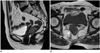 | Fig. 7Rectal schwannoma in 67-year-old woman.
Sagittal T2-weighted image (A) and axial T1-weighted image (B) show well-circumscribed homogeneous submucosal mass (arrows) in lower rectum.
|
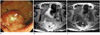 | Fig. 8Primary rectal lymphoma in 62-year-old man.
Optical colonoscopy (A) identified fungating mass (black arrows) with central necrosis (white arrowhead). Axial T2-weighted image (B) and T1-weighted image (C) show 4.5-cm well circumscribed fungating mass (arrows) with central ulceration (arrowheads) located at upper rectum. Homogeneous tumoral signal intensity and preservation of fat planes may suggest lymphoma.
|
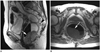 | Fig. 9Malignant melanoma in 66-year-old woman.
Sagittal T2-weighted image (A) and axial T1-weighted image (B) show bulky intraluminal fungating mass (arrows) involving distal rectum. Perirectal infiltration and necrotic nodal metastases (arrowhead) in posterior side of mid rectum are seen. Signal intensity of this mass is heterogeneously high on T2-weighted image and low on T1-weighted image. After performing Mile's operation, amelanocytic melanoma with lymph node metastases was confirmed.
|
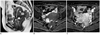 | Fig. 10Endometriosis that presented as rectal submucosal mass in 46-year-old woman.
Sagittal T2-weighed image (A) shows areas of irregular tissue signal intensity in rectouterine pouch with internal cyst-like structure (arrowhead). Infiltrative rectal wall thickening (arrows) suggests rectal wall involvement. This cyst was identified as hemorrhagic structure by high signal intensity after fat suppression on axial T1-weighted image (arrowhead) (B). Gadolinium enhanced axial T1-weighted image with fat suppression (C) demonstrates heterogeneous enhancing infiltrative mass involving anterior rectal wall and rectouterine pouch (arrows). Lower anterior resection was performed, and endometriosis involvement in submucosa, proper muscle and subserosa of rectum was diagnosed.
|
 | Fig. 11Tailgut cyst in 47-year-old woman.
Sagittal T2-weighted image (A) shows multilocular cystic mass with variable signal intensities (arrows) posterior to rectum (arrowheads). Gadolinium-enhanced axial T1-weighted image with fat suppression (B) also shows thin peripheral cystic wall enhancement without enhancing solid portions (arrow). On sectioning (not shown), surgical specimen revealed brownish friable material and microscopic examination demonstrated mucin-containing columnar epithelium, which is consistent with tailgut cyst.
|
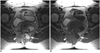 | Fig. 12Adenocarcinoma arising from tailgut cyst in 40-year-old woman.
Axial T2-weighted image (A) and axial T1-weighted image (B) revealed infiltrative soft tissue mass (arrowheads) involving right gluteus muscle and coccygeal area. Retrorectal multilocular cystic mass is adjacent to this infiltrative mass (arrows). Histopathologic diagnosis was poorly-differentiated adenocarcinoma arising from tailgut cyst.
|
 | Fig. 13Duplication cyst in 28-year-old woman.
Axial T2-weighted image (A) and gadolinium enhanced axial T1-weighted image with fat suppression (B) show retrorectal unilocular cystic structure with relatively thick cystic wall enhancement (white arrows) that is displacing rectum (white arrowhead). There was no communication between lesion and rectal lumen on barium study (not shown). Peripheral calcifications are seen as focal dark signal intensities (black arrowheads), which were confirmed by CT. However, calcification in alimentary duplication cyst is extremely rare finding. Microscopic assessment after surgical excision (C) revealed ectopic muscle layer (white arrow) located at subserosal compartment of rectum (black arrowhead: rectal mucosa). Superficial to this muscle layer lies submucosa and mucosa (black arrow) layer of cystic lesion.
|
 | Fig. 14Epidermoid cyst in 31-year-old woman.
Oblique coronal T2-weighted image (A) shows thin-walled unilocular cystic lesion (arrows) lateral to rectum. Note small linear hypointense structures (arrowheads) that possibly represent keratin aggregates. Gadolinium enhanced axial T1-weighted image with fat suppression (B) revealed mild enhancement of cystic wall (arrows) without evidence of enhancing soft tissue components.
|
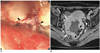 | Fig. 15Ovarian cancer mimicking rectal intramural submucosal tumor in 44-year-old woman who recently developed hematochezia.
Optical colonoscopy (A) demonstrates large intramural submucosal tumor-like lesion with central ulceration (black arrowheads). Gadolinium enhanced axial T1-weighted image with fat suppression (B) shows 4-cm mass (white arrows) with large central ulceration (black arrowheads). Possibility of intramural mesenchymal tumors such as gastrointestinal stromal tumor was suggested on MRI interpretation. However, presence of enlarged lymph node in left pelvic side wall (black arrow), indicating possible nodal metastases, is inconsistent with this diagnosis since nodal metastasis is extremely rare in gastrointestinal stromal tumor cases. After operation, diagnosis was poorly-differentiated serous adenocarcinoma arising from right ovary with direct invasion into rectum.
|
References
1. Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 1. Neoplasms. Radiographics. 2007. 27:1681–1692.
2. Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 2. Nonneoplastic causes. Radiographics. 2007. 27:1693–1703.
3. Rouse HC, Godoy MC, Lee WK, Phang PT, Brown CJ, Brown JA. Imaging findings of unusual anorectal and perirectal pathology: a multi-modality approach. Clin Radiol. 2008. 63:1350–1360.
4. Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004. 1014:13–27.
5. Okamoto Y, Fujii M, Tateiwa S, Sakai T, Ochi F, Sugano M, et al. Treatment of multiple rectal carcinoids by endoscopic mucosal resection using a device for esophageal variceal ligation. Endoscopy. 2004. 36:469–470.
6. Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005. 37:630–634.
7. Grabowski P, Schonfelder J, Ahnert-Hilger G, Foss HD, Heine B, Schindler I, et al. Expression of neuroendocrine markers: a signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002. 441:256–263.
8. Chang S, Choi D, Lee SJ, Lee WJ, Park MH, Kim SW, et al. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007. 27:1667–1679.
9. Heah SM, Eu KW, Ooi BS, Ho YH, Seow-Choen F. Tumor size is irrelevant in predicting malignant potential of carcinoid tumors of the rectum. Tech Coloproctol. 2001. 5:73–77.
10. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002. 33:459–465.
11. Grassi N, Cipolla C, Torcivia A, Mandala S, Graceffa G, Bottino A, et al. Gastrointestinal stromal tumour of the rectum: report of a case and review of literature. World J Gastroenterol. 2008. 14:1302–1304.
12. Darnell A, Dalmau E, Pericay C, Musulen E, Martin J, Puig J, et al. Gastrointestinal stromal tumors. Abdom Imaging. 2006. 31:387–399.
13. Maciejewski A, Lange D, Wloch J. Case report of schwannoma of the rectum--clinical and pathological contribution. Med Sci Monit. 2000. 6:779–782.
14. Lee SH, Kim TO, Hwang SY, Ryu DY, Lee DH, Park WI, et al. [A case of rectal schwannoma presenting with hematochezia]. Korean J Gastroenterol. 2006. 48:195–199.
15. Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961. 49:80–89.
16. Ghai S, Pattison J, O'Malley ME, Khalili K, Stephens M. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. 2007. 27:1371–1388.
17. Dionigi G, Annoni M, Rovera F, Boni L, Villa F, Castano P, et al. Primary colorectal lymphomas: review of the literature. Surg Oncol. 2007. 16:Suppl 1. S169–S171.
18. Sashiyama H, Takayama W, Miyazaki S, Makino H, Matsushita K, Shimada H, et al. The diagnostic value of endoscopic ultrasonography and magnetic resonance imaging for anorectal malignant melanoma: report of a case. Surg Today. 2003. 33:209–213.
19. Kim KW, Ha HK, Kim AY, Kim TK, Kim JS, Yu CS, et al. Primary malignant melanoma of the rectum: CT findings in eight patients. Radiology. 2004. 232:181–186.
20. Matsuoka H, Nakamura A, Iwamoto K, Sugiyama M, Hachiya J, Atomi Y, et al. Anorectal malignant melanoma: preoperative usefulness of magnetic resonance imaging. J Gastroenterol. 2005. 40:836–842.
21. Caramella T, Novellas S, Fournol M, Bafghi A, Mondot L, Chassang M, et al. [Deep pelvic endometriosis: MRI features]. J Radiol. 2008. 89:473–479.
22. Aflalo-Hazan V, Rousset P, Mourra N, Lewin M, Azizi L, Hoeffel C. Tailgut cysts: MRI findings. Eur Radiol. 2008. 18:2586–2593.
23. Yang DM, Yoon MH, Kim HS, Oh YH, Ha SY, Oh JH, et al. Presacral epidermoid cyst: imaging findings with histopathologic correlation. Abdom Imaging. 2001. 26:79–82.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download