Abstract
Objective
We wanted to investigate the usefulness of event-related (ER) functional MRI (fMRI) for the assessment of cortical visual impairment in infants with periventricular leukomalacia (PVL).
Materials and Methods
FMRI data were collected from 24 infants who suffered from PVL and from 12 age-matched normal controls. Slow ER fMRI was performed using a 3.0T MR scanner while visual stimuli were being presented. Data analysis was performed using Statistical Parametric Mapping software (SPM2), the SPM toolbox MarsBar was used to analyze the region of interest data, and the time to peak (TTP) of hemodynamic response functions (HRFs) was estimated for the surviving voxels. The number of activated voxels and the TTP values of HRFs were compared. Pearson correlation analysis was performed to compare visual impairment evaluated by using Teller Acuity Cards (TAC) with the number of activated voxels in the occipital lobes in all patients.
Results
In all 12 control infants, the blood oxygenation level-dependent (BOLD) signal was negative and the maximum response was located in the anterior and superior part of the calcarine fissure, and this might correspond to the anterior region of the primary visual cortex (PVC). In contrast, for the 24 cases of PVL, there were no activated pixels in the PVC in four subjects, small and weak activations in six subjects, deviated activations in seven subjects and both small and deviated activations in three subjects. The number of active voxels in the occipital lobe was significantly correlated with the TAC-evaluated visual impairment (p < 0.001). The mean TTP of the HRFs was significantly delayed in the cases of PVL as compared with that of the normal controls.
Infants with cerebral palsy (CP) frequently present with cortical visual impairment (CVI) that is often caused by damage to the retrochiasmatic pathways (1). This is particularly true for patients with damage to the periventricular white matter. Periventricular leukomalacia (PVL) is the leading cause of chronic motor disability in infants after hypoxic insult, and this is due to an ischemic infarction of the periventricular white matter, which is the vascular watershed zone in the developing fetus (2). The visual fields are thought to be affected early in the course of the disease.
Severe CVI with perinatal brain lesions can be found even in the presence of normal visual acuity testing (3). In addition, peripheral visual field testing is necessary to define the cortical visual impairment, which is impossible before three years of age (4). However, the maximum plasticity of visual function occurs within the first two years of age (5). Therefore, to provide an accurate prognosis and appropriately targeted interventions for infants with CVI, new methods are needed for defining the anatomic and functional basis of CVI early in development.
Blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) is currently used to map brain functions (6). On the basis of the local changes in blood oxygenation, the fast MR imaging sequences can be used to visualize the regional neuronal activity elicited by a given task. This technique offers the ability to non-invasively study the response of the visual cortex upon photic stimulation in normally developing children and in children with structural lesions of the visual pathways.
However, during fMRI examination, the patients are required to remain motionless and to understand and perform the given task. These requirements have restricted the use of fMRI in infants and young children in general, and the degree of cooperation required may be particularly difficult for children with coexisting intellectual impairment. The development of passive paradigms and the use of sedatives should avoid these obstacles. Fortuitously, visual stimuli do not require patient cooperation because they can be passively presented.
Sie et al. (7) performed fMRI research on 21 infants with PVL using a 1.5T MR scanner. Although the visual responses of these 21 infants were comparable to those of healthy control infants, the activated cortical volume in the visual cortex of the injured infants was shown to be highly correlated with the severity of occipital PVL. The signal-to-noise ratio (SNR) of BOLD contrast is higher on 3T scanners than on 1.5T scanners; thus, a 3T scanner may be sensitive enough to detect a small difference in the BOLD response in the visual cortex of both control and PVL infants.
The purpose of our study was to investigate the event-related (ER) fMRI signals for the assessment of CVI in sedated PVL infants with using passive visual tasks and a 3T MR scanner.
In this prospective hospital-based study, the subjects were 37 infants (F:M = 15:22; age range, 0.6-1.5 years; median age, 1.0 years) B and this group included 18 pre-term infants (gestational age: 30.5 ± 2.1 weeks) and 19 full-term infants with cerebral palsy. All patients had a history of hypoxic insult followed by coma or altered consciousness with or without convulsions in the neonatal period. Those infants with visual impairment caused by ophthalmological abnormalities were excluded. Complete ophthalmological examinations were performed and the binocular visual acuity was also evaluated using Teller Acuity Cards (TAC). In addition, 16 age-matched normal controls (F:M = 7:9; age range, 0.5-1.5 years; median age, 1.0 years) with normal MRI findings and without neurological abnormalities were recruited for comparison. Written informed consent was obtained from all of the participants' parents or legal guardians, and all procedures were performed according to the guidelines of the institutional review board for clinical studies.
All infants were sedated for 45 min before imaging with 30 mg/kg of chloral hydrate, and they were quietly asleep during the examination. During the scan, the arterial oxygen saturation and heart rate were monitored in all of the infants, adhesive earmuffs were added for ear protection and the infants' heads were immobilized using a polystyrene bead-filled pillow from which the air was evacuated.
The fMRI scans were performed using a 3.0T MR scanner (Intera Achieva, Philips Medical Systems, The Netherlands) with an 8-channel sensitivity-encoding (SENSE) head coil.
Functional images were obtained using a gradient-echo echo-planar image (EPI) sequence (TR [repetition time]/TE [echo time] = 2000/30 ms, field of view [FOV] = 220 mm, matrix = 64 × 64, 4-mm axial slices). Functional scanning was always preceded by 8 sec of dummy scans to ensure tissue steady-state magnetization. Anatomical scans were performed after the fMRI scans. A fast field echo (FFE) T1-weighted sequence (TR/TE = 2500/15 ms, FOV = 220 mm, matrix = 512 × 512, thickness = 4 mm) was performed to acquire the same volume as in the functional session.
For the first experiment, a block paradigm that alternated between "control" and "activation" sequences was applied. A 2-Hz visual flash was applied during the activation period. The visual stimuli were applied at a relatively low frequency in accordance with the stimulation frequency used for the visually evoked potentials in newborns and infants prior to myelination of the optic pathway. After the first control block (30 sec), the two conditions (30 sec each) were repeated four times, yielding a total duration of 270 sec per stimulation run.
The last experiment was done using the slow event-related approach (8). A constant inter-stimulus interval was used to avoid an assumption about the linearity of the BOLD signal in newborns (9) and to improve the detection accuracy (10). The stimulus (2-Hz visual flash) was presented for 5 sec. The inter-stimulus interval was 60 sec. Stimulus presentation was repeated 10 times, leading to scan duration of 11 min with a total MRI scan time of approximately 27 min for each child. The infants' eyes remained closed throughout the examination.
Preprocessing and statistical analysis of the fMRI data were performed using the SPM2 software package (Welcome Department of Cognitive Neurology, London, UK) and using the Matlab platform. The images were realigned and subsequently corrected for differences in the slice acquisition time using temporal realignment to the middle slice. If the head motion in the fMRI data was > 2 mm, then the data was discarded.
The images were then normalized to the standard stereotactic space corresponding to the template from the post-processing of pediatric MR images of 2-year-old children download from Hammersmith Hospital of the Imperial College, London (http://www.doc.ic.ac.uk/~dr/brain-development). This was performed using the SPM Toolbox VBM2 v1.09 (dbm.neuro.uni-jena.de/vbm) and smoothing with an isotropic 6-mm Gaussian kernel. A voxel-by-voxel comparison according to the general linear model (GLM) was used to calculate the activation differences between the active and resting conditions.
Significant signal changes for each contrast were assessed using F-statistics on a voxel-by-voxel basis. The resulting set of voxel values for each contrast constituted statistical parametric mapping (SPM) of the F-statistic. The threshold was set to p values < 0.05 and familywise error (FWE) was corrected for the single-subject analysis. The activated images were superimposed on the standardized T1-weighted images and the single-subject EPI and T1-weighted images. The coordinates of the maximum response and the number of activated voxels in the occipital lobe were recorded.
The quantitative analysis of the ER fMRI data was performed using the regions of interest (ROI) method centered on the activated areas in the occipital lobe. The SPM toolbox MarsBar (MRC Cognition and Brain Sciences Unit, Cambridge, UK) was used to engage the statistical analyses of the ROI data, and the hemodynamic response functions (HRFs) for both the patients and the normal controls in the ER paradigm were obtained. The post-stimulus onset BOLD signal time to peak (TTP) of the HRF was measured. The number of activated voxels and the TTPs of the HRF were analyzed by Student's t test. A p value < 0.05 indicated statistical significance. The Pearson correlation analysis test was performed to compare visual impairment with the number of activated voxels in the occipital lobes of all PVL infants.
Four PVL infants (2 full-term and 2 pre-term) and one control infant awakened before or during the fMRI scan, and the head motions of nine PVL infants (5 full-term and 4 pre-term) and three controls were > 2 mm. The fMRI data of these 17 infants (13 PVL and 4 controls) were discarded.
The fMRI data of 24 PVL infants (age range, 0.6-1.4 years; median age, 1.2 years), including 12 pre-term (gestational age, 30.5 ± 2.1 weeks) and 12 full-term infants, and 12 normal controls (age range, 0.6-1.5 years, median age, 1.1 years) were included. Of the PVL infants, 18 (75%) presented with visual impairment. Of these, six (33%) were totally or almost totally blind and 12 (67%) had low vision. The other six PVL infants (24%) had normal (4) or near-normal (2) vision.
Of the 12 normal infants, the BOLD response to the stimuli presented as a signal decrease. Signal fluctuations after the return to baseline and an overshoot were observed in both the patients and controls. The maximum response was located in the anterior and superior part of the calcarine fissure, and this may correspond to the anterior region of the primary visual cortex (PVC) (Fig. 1). However, of the 24 infants with PVL, there were no activated voxels in four cases, tiny activations (< 1500 voxels) in six cases (Figs. 2, 5), deviated activations (in which the maximum response was not in the anterior or superior part of the calcarine fissure) in seven cases (Fig. 3), and both tiny and deviated activations in three cases (Fig. 4).
The number of active voxels in the occipital lobe of the PVL infants (1544.9 ± 779.0) was significantly lower than that of the controls (2335.8 ± 672.6), and fewer active voxels were found in the pre-term PVL infants (685.1 ± 122.2) as compared with that of the full-term infants (2000.2 ± 427.2). The number of active voxels in the occipital lobe was significantly correlated with visual impairment as evaluated by TAC (p < 0.001) (Fig. 6). The mean TTP of the HRFs was significantly delayed in the PVL infants as compared with that of the normal controls (4.67 ± 0.98 versus 8.84 ± 1.33, respectively, p < 0.05). Additionally, the TTPs significantly differed between the pre-term and full-term infants (7.83 ± 1.33 versus 9.85 ± 2.48, respectively, p < 0.05).
Cortical visual impairment is the leading cause of bilateral visual impairment, which is defined as a visual deficit that is caused by disturbance of the posterior visual pathways, including the optic radiations, the striate cortex and/or the prestriate cortex. CVI remains difficult to clinically assess in young infants. A high correlation between the degree of PVL and the severity of CVI has been documented in many studies using conventional MRI and clinical visual acuity testing (11). However, clinical measurement of visual field defects is difficult and even in normally developing children.
The potential of fMRI has been pointed out in studies involving patients with visual deficits. However, to date, few functional studies of visual perception have been performed in infants and children suffering from CVI. In this study, we focused on PVL infants, with or without visual impairments, to quantitatively assess the destruction of the involved posterior visual pathway.
Functional activation of the normal controls was localized in the medial occipital lobe. The site of maximal activity was primarily the anterior calcarine sulcus, which may correspond to projections of the peripheral visual field and that are received by the anterior region of the PVC. This activity may be related to the manner in which the stimuli were presented. Visual stimulation was given through closed eyes in the absence of a fixation point or the control of eye position. Therefore, only the peripheral visual field may have been excited (12). Furthermore, the use of a relatively low stimulus rate (2 Hz, in accordance with visual evoked-potential studies (13)) may be a factor in our findings.
Negative BOLD responses were found in our study. Their shape is similar to a negative mirror image of the known positive adult BOLD response. Negative BOLD responses have been observed by different research groups in infants and children, and specifically in those infants older than eight weeks (14) or those children between four and 40 months of age (15). Negative BOLD responses have also been reported in the primary auditory cortex in sedated and non-sedated infants (15). The explanation of the negative BOLD response in infants may reflect a rapid increase in the deoxyhemoglobin/oxyhemoglobin ratio, which is likely to be caused by impaired cerebral blood flow autoregulation or by high augmentation of the oxygen consumption.
The results of the block paradigm demonstrate that the activation size in PVL infants was smaller than that in the normal controls, and the number of voxels activated during fMRI was well-correlated with the clinical visual acuity results. This result might be explained by vascular incompetence, altered BOLD phenomena or cortical non-function. This aberrant or deviated fMRI activation may suggest the occurrence of cortical reorganization with conserved visual function. Despite that PVL causes substantial structural deformities of the occipital cortex in some patients, visual function plasticity may have occurred when considering their very young age. Reorganization may compensate, to some degree, for the PVL-induced visual impairment, which may lead to an optimistic potential for visual development in some children affected by cerebral palsy caused by PVL, as was reported by Porro et al. (16).
The results of our event-related fMRI paradigm showed that the TTP of the HRFs was significantly delayed in the cases of PVL as compared with that of the normal controls, and even in patients with normal or near-normal visual acuity. The TTP delay may reflect white matter injury in the posterior visual pathways, leading to impaired input from the retina to the PVC. The delay may be more sensitive than the decay of the BOLD signal for predicting some diseases such as early Alzheimer disease (17). However, considering the limited sample size of our study, the HRF variability across subjects (18) and the shifted neural pathway due to functional reorganization (19), we did not perform further analysis of the correlation between the TTP delay and white matter injury.
Chloral hydrate was administered to sedate all infants to ensure better fMRI success rates. Previous studies have ruled out the influence of sedation on the BOLD signal characteristics (14, 15). We used low doses of chloral hydrate (20) and we performed the scans 45 min after administering chloral hydrate (14). Therefore, any influence of sedation on our data was greatly diminished. However, more experimental data is needed before definitive conclusions can be drawn concerning the decreased BOLD signal in infants.
The clinical relevance of injury and the related modification of the above-mentioned white matter architecture detected in this study remain unknown, so long-term follow-up studies will be necessary to clarify the relationship between altered white matter anatomy and the outcome of clinical visual impairment (21). In particular, the correlations between damage to different cortical and/or subcortical connections and the types of neuropsychological deficits require further study.
In conclusion, we demonstrated the feasibility of using fMRI to investigate the visual impairment in children with PVL. Decreases in the number of active voxels in the occipital lobe and a TTP delay of the HRFs in these children may be indicators of visual impairments in children with PVL.
Figures and Tables
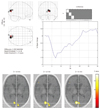
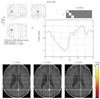
Fig. 1
Active area and hemodynamic response function of normal infant.
Hemodynamic response function is located in upper right hand corner of active map.
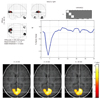
Fig. 2
Active area and hemodynamic response function of full-term primary visual cortex infant.
Active area is slightly decreased, time to peak is slightly prolonged and result of Teller Acuity Card examination is rated "+-".
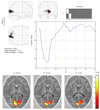
Fig. 3
Active area and hemodynamic response function of full-term periventricular leukomalacia infant.
Active area is deviated in nature, time to peak is prolonged and result of Teller Acuity Card examination is rated "+-".
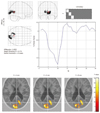
Fig. 4
Active area and hemodynamic response function of pre-term periventricular leukomalacia infant.
Active area is tiny and deviated, time to peak is slightly prolonged and result of Teller Acuity Card examination is rated "+".
References
1. van den Hout BM, de Vries LS, Meiners LC, Stiers P, van der Schouw YT, Jennekens-Schinkel A, et al. Visual perceptual impairment in children at 5 years of age with perinatal haemorrhagic or ischaemic brain damage in relation to cerebral magnetic resonance imaging. Brain Dev. 2004. 26:251–261.
2. Fan GG, Yu B, Quan SM, Sun BH, Guo QY. Potential of diffusion tensor MRI in the assessment of periventricular leukomalacia. Clin Radiol. 2006. 61:358–364.
3. Mercuri E, Atkinson J, Braddick O, Anker S, Cowan F, Rutherford M, et al. Basal ganglia damage and impaired visual function in the newborn infant. Arch Dis Child Fetal Neonatal Ed. 1997. 77:F111–F114.
4. Curnyn KM, Kaufman LM. The eye examination in the pediatrician's office. Pediatr Clin North Am. 2003. 50:25–40.
5. Braddick O, Atkinson J, Hood B, Harkness W, Jackson G, Vargha-Khadem F. Possible blindsight in infants lacking one cerebral hemisphere. Nature. 1992. 360:461–463.
6. Kim GW, Jeong GW, Kim TH, Baek HS, Oh SK, Kang HK, et al. Functional neuroanatomy associated with natural and urban scenic views in the human brain: 3.0T functional MR imaging. Korean J Radiol. 2010. 11:507–513.
7. Sie LTL, Rombouts SA, Valk IJ, Hart AA, Scheltens P, van der Knaap MS. Functional MRI of visual cortex in sedated 18 month-old infants with or without periventricular leukomalacia. Dev Med Child Neurol. 2001. 43:486–490.
8. Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997. 5:329–340.
9. Bandettini PA, Cox RW. Event-related fMRI contrast when using constant interstimulus interval: theory and experiment. Magn Reson Med. 2000. 43:540–548.
10. Flodmark O, Lupton B, Li D, Stimac GK, Roland EH, Hill A, et al. MR imaging of periventricular leukomalacia in childhood. AJR Am J Roentgenol. 1989. 152:583–590.
11. Altman NR, Bernal B. Brain activation in sedated children: auditory and visual functional MR imaging. Radiology. 2001. 221:56–63.
12. Pike AA, Marlow N, Reber C. Maturation of the flash visual evoked potential in preterm infants. Early Hum Dev. 1999. 54:215–222.
13. Morita T, Kochiyama T, Yamada H, Konishi Y, Yonekura Y, Matsumura M, et al. Difference in the metabolic response to photic stimulation of the lateral geniculate nucleus and the primary visual cortex of infants: a fMRI study. Neurosci Res. 2000. 38:63–70.
14. Martin E, Joeri P, Loenneker T, Ekatodramis D, Vitacco D, Hennig J, et al. Visual processing in infants and children studied using functional MRI. Pediatr Res. 1999. 46:135–140.
15. Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001. 19:1–5.
16. Porro G, Wittebol-Post D, Van Nieuwenhuizen O, Schenk Rootlieb AJ, Treffers WF. Longitudinal follow-up of grating acuity in children affected by cerebral palsy: results of a 5 year study. Eye (Lond). 1998. 12(Pt 5):858–862.
17. Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer's disease. Neuroimage. 2005. 26:1078–1085.
18. Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004. 21:1639–1651.
19. Sydekum E, Baltes C, Ghosh A, Mueggler T, Schwab ME, Rudin M. Functional reorganization in rat somatosensory cortex assessed by fMRI: elastic image registration based on structural landmarks in fMRI images and application to spinal cord injured rats. Neuroimage. 2009. 44:1345–1354.
20. Seghier ML, Lazeyras F, Zimine S, Maier SE, Hanquinet S, Delavelle J, et al. Combination of event-related fMRI and diffusion tensor imaging in an infant with perinatal stroke. Neuroimage. 2004. 21:463–472.
21. Kim EY, Park HJ, Kim DH, Lee SK, Kim J. Measuring fractional anisotropy of the corpus callosum using diffusion tensor imaging: mid-sagittal versus axial imaging planes. Korean J Radiol. 2008. 9:391–395.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download