Abstract
This paper reports on issues relating to the optimal use of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging (Gd-EOB-DTPA MR imaging) together with the generation of consensus statements from a working group meeting, which was held in Seoul, Korea (2010). Gd-EOB-DTPA has been shown to improve the detection and characterization of liver lesions, and the information provided by the hepatobiliary phase is proving particularly useful in differential diagnoses and in the characterization of small lesions (around 1-1.5 cm). Discussion also focused on advances in the role of organic anion-transporting polypeptide 8 (OATP8) transporters. Gd-EOB-DTPA is also emerging as a promising tool for functional analysis, enabling the calculation of post-surgical liver function in the remaining segments. Updates to current algorithms were also discussed.
Recent advances in imaging techniques may enable earlier and more accurate diagnosis of hepatocellular carcinoma (HCC), with the ultimate aim of improving its poor prognosis. To date, HCC is the third most common cause of cancer deaths worldwide (1), and the survival rate in developing countries is only around 3-5% (2). As imaging modalities evolve, so do the potential benefits; for example, volumetric (3D) ultrasound (US) could increase diagnostic accuracy by enabling a high signal-to-noise ratio; contrast-enhanced (Sonazoid® or Sonovue®) US may improve vascularity assessment; and dual-energy computed tomography (CT) could have potential clinical application in non-contrast imaging, including the assessment of liver, iron and fat content of biliary stones; whilst perfusion CT could differentiate diverse tumor tissue, but the radiation dosage needed (10-20 mSV) is likely to limit its applicability.
The role of contrast-enhanced magnetic resonance imaging (CE-MR imaging) with agents such as GD-EOB-DTPA (Primovist®; Bayer HealthCare, Berlin, Germany) is expanding, with this modality being widely viewed as a problem-solving tool. The biphasic nature of Gd-EOB-DTPA enables dynamic-phase imaging, and as approximately 50% is taken up by organic anion-transporting polypeptide 8 (OATP8) (also referred to as OATP1B3) receptors in functioning liver hepatocytes, this also enables images to be obtained in the hepatobiliary phase (3-8). Gd-EOB-DTPA MR imaging is a comprehensive liver analysis tool; this is largely due to the hepatocellular uptake, subsequent biliary excretion and hemodynamic qualities of this contrast agent. These features are expected to: improve tumor detection and characterization compared with other imaging modalities; enable small lesions to be monitored over a long-term follow-up period; increase the diagnostic accuracy of post-resection liver-related complications, and the anatomical and functional information obtained from Gd-EOB-DTPA MR imaging can be used for planning treatment decisions.
It is also speculated that hepatobiliary agents may be used to evaluate the progressive loss of the biliary polarization, and the impairment of the microstructure of the biliary secretion (9). This, and other pathological changes that occur during hepatocarcinogenesis can be captured with CE-MR imaging, and it is essential to optimize the role of Gd-EOB-DTPA MR imaging in the evaluation of this process, particularly during early stages of HCC development.
Some of these issues were considered at the 4th International Forum for Liver MR imaging (Primovist® User Meeting), which was held in Seoul, South Korea, October 29-30, 2010. Approximately 90 delegates from Europe, Asia and the USA attended the meeting; all delegates participated in the generation of statements on the role of CE-MR imaging and Gd-EOB-DTPA using an interactive system. Statements were defined in workgroups and those statements that were supported by the majority of the delegates are reported in this paper.
The detection and characterization of small HCCs (≤ 1.5 cm) is one of the most important, but challenging issues in the management of cirrhotic patients. Small HCCs can be divided into early HCCs, which have a vaguely nodular appearance and are well differentiated, whereas progressed HCCs have a distinctly nodular pattern, are moderately differentiated with evidence of intra-hepatic spread (10, 11). Early HCCs grow more slowly than progressed HCCs and have a more favorable outcome both in terms of time to recurrence and long-term survival rate (12). However, the transition from pre-malignant dysplastic nodules (DNs) to early and progressed HCCs can be difficult to determine. This transition is heterogeneous; and not all nodules display step-wise progression, which may be due to variation in the developmental process (13).
There is evidence to suggest that lesion size can be a good indicator of typical multistep development. In a study of 980 nodules resected from 664 patients, the incidence of multistep hepatocarcinogenesis was 74% in tumors ≤ 1 cm and was 9% in tumors > 5 cm (14). The transition from high-grade DN (HGDN) to early HCC (grade I [little cellular atypia]) is supposed to generally occur in lesions < 1 cm in size. This transition may be histopathologically characterized by the appearance of grade I foci in DN (Fig. 1A). Further progression of early HCC to nodule-in-nodule type HCC (grade II [overt carcinoma nodule in early HCC]) may occur in lesions around 1.5 cm, and this transition may be initially characterized by grade II foci appearing in grade I lesions (Fig. 1B). The transformation from slow to rapid growing HCCs also occurs in lesions around 1.5 cm in size. A follow-up study of 53 nodules has shown that the tumor volume doubling time was 13.9 (11.7) for early HCC and 6.0 (5.2) months for progressed HCC; distribution of lesions based on these data are summarized in Figure 2 (15, 16).
Consensus statement: Biopsy or follow-up should be considered in small (i.e. 1-1.5 cm) hypointense lesions in the hepatobiliary phase of Gd-EOB-DTPA.
The use of contrast-enhanced MR imaging with agents such as Gd-EOB-DTPA could improve the detectability rate of early HCC (in nodules around 1-1.5 cm). In a study of 30 resected specimens, a low- to slightly low-signal intensity in the hepatobiliary phase of Gd-EOB-DTPA MR imaging correctly identified 23 of 24 hypovascular hepatocyte nodules as early HCCs (only 1 nodule was wrongly identified as a DN or a regenerative nodule [RN]). An iso- to high-signal intensity correctly identified 5 of 6 lesions as a DN or RN (only one nodule was wrongly identified as early HCC). The detection accuracy was 93% (28 of 30) in this study (17). Additional information from histopathological samples (i.e. biopsy samples and genetic biomarkers such as HSP70, CAP2, GPC3, beta-catenin and p53) (13) could provide detailed information about the grade of malignancy for each nodule. Taken together, this approach could enable more detailed information on patient outcome and treatment choice at an early stage.
Consensus statement: The hepatobiliary phase of Gd-EOB-DTPA reflects early hepatocarcinogenesis.
In addition to tumor size, OATP8 expression could also correlate with differentiation and histopathological features of liver lesions. OATP8 is a member of the OATP family, which transport both intrinsic factors (such as bile acid components and hormones) and extrinsic factors (such as drugs) throughout the liver, kidney and gastrointestinal tract (18). Unlike other members of the family, OATP8 expression is restricted to the basolateral and lateral membrane of human hepatocytes (19). Another member of the family, OATP-C also mediates the uptake of amphipathic compounds into hepatocytes; this transporter exists on the sinusoidal side of hepatocytes.
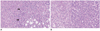
However, the liver-enriched transcription factor HNF3beta has been found to repress the transcription of OATP8, but not OATP-C in HCCs; HNF3beta was increased in 70% of lesions and correlated inversely with OATP8 mRNA. As a result, the expression of OATP8 was decreased by 60% in the HCC compared with the surrounding normal parenchyma, but expression of OATP-C was not significantly decreased (20). These findings have been confirmed by immunohistochemical staining of 49 HCCs resected from 47 patients using a primary antibody against human OATP8 (7). This was followed by semiquantitative evaluation of OATP8 intensity. The degree of OATP8 expression was found to correlate with tumor differentiation in hypointense HCC (Fig. 3).
Organic anion-transporting polypeptide 8 is the most probable uptake transporter for Gd-EOB-DTPA in human hepatocytes, and it is likely to be excreted into bile secretions by multi-drug resistance proteins (MRP), predominantly MRP3 (7, 8, 21). Signal intensity during the hepatobiliary phase of Gd-EOB-DTPA is, therefore, likely to correlate with expression of these molecular transporters. As the degree of OATP8 expression declines during multi-step hepatocarcinogenesis according to malignancy grade, there will be a decline of enhancement ratio on the hepatobiliary phase due to a reduced Gd-EOB-DTPA uptake. Calculation of this enhancement ratio could be useful for estimating malignancy grade (except in around 5-10% of HCCs, that show prominent expression of OATP8, which is probably due to genetic alterations). It is also important to note that the signal intensity on the hepatobiliary phase of Gd-EOB-DTPA differs between hypo- and iso-/hyper-intense HCCs (Fig. 4); this has also been confirmed by immunostaining. This may be due to the fact that hypointense HCCs have lower expression of OATP8 (7).
Use of extracellular contrast media (ECCM) for dynamic MR imaging provides sufficient information for confident diagnosis of typical enhancing HCC, but for hypovascular lesions and mimickers, more information is required. This 'gap' has traditionally been filled by fine needle biopsy and immunohistochemical analysis, however, the extra information provided by dual contrast agents such as Gd-EOB-DTPA, with both dynamic and hepatobiliary phase imaging (HBPI) characteristics, may allow a more detailed and confident diagnosis of cirrhotic liver lesions from one MR imaging procedure. Such advances in contrast media may have enabled the number of imaging modalities needed for a correct diagnosis to be decreased, and this may have reduced the need for fine-needle biopsies (22).
These advances may also be reflected in the changing guidelines; the AASLD (2005) guidelines recommended that coincidental, conclusive imaging features on two dynamic imaging tests were required to make a diagnosis of HCC for US-defined nodules of between 1-2 cm diameter (23). However, in the updated guidelines, only one dynamic imaging test is recommended (either 4-phase multidetector computed tomography [MDCT] or CE-MR imaging), and if atypical imaging features are present, then both modalities can be used (24).
Consensus statement: GD-EOB-MR imaging can be used as a first-line tool for the characterization of a new nodule that has been detected in cirrhotic liver using US (including contrast-enhanced ultrasound [CEUS]. Gd-EOB-MR imaging can also replace ECCM MR imaging for this purpose.
The updated guidelines on HCC detection and diagnosis from the Japan Society of Hepatology have include HBPI information in their algorithm as a problem-solving tool when dynamic MR imaging or CT show atypical vascular features (25). The benefits of HBPI include a homogeneous, strong and prolonged enhancement of liver parenchyma through cellular uptake, resulting in good contrast between focal liver lesions and healthy parenchyma. The typical enhancement pattern achieved, which is considered the hallmark of an HCC, is the presence of arterial enhancement followed by washout of the tumor in the portal venous and/or delayed phases (26). Data from 55 patients with 66-pathology confirmed HCCs showed that a diagnosis (defined as arterial enhancement plus washout) could be established in 65% patients with CT and in 80% with Gd-EOB-DTPA MR imaging. When hypointensity of nodule on HBPI was added to the diagnostic criteria (as most HCCs nodules will show this), 83% were diagnosed as HCCs using Gd-EOB-DTPA MR imaging (27) (Fig. 5).
Consensus statement: Gd-EOB-DTPA is an appropriate second-line imaging modality as recommended by the recent Japanese guidelines.
Contrast-enhanced MR imaging also shows a higher sensitivity for HCC detection, especially for nodules < 2 cm diameter compared with other imaging modalities (28-30). The information provided by the hepatobiliary phase of Gd-EOB-DTPA MR imaging may increase the diagnostic accuracy of small HCC compared with MDCT; the mean sensitivity of dynamic and HB images was significantly higher (0.72) than dynamic MR images alone (0.63; p = 0.008) or MDCT (0.61; p = 0.001) in a sample of 67 HCCs from 36 patients (31). HBPI also allows greater differentiation of small hypervascular HCCs (≤ 2 cm) from arterially enhancing pseudolesions (AEPs) (32, 33). In a study of 69 patients with 53 AEPs and 44 HCCS; 95% of HCCs demonstrated low signal intensity in the hepatobiliary phase and 94% of AEPs showed iso-signal intensity on the hepatobiliary phase (32).
In addition, HBPI may provide information regarding hepatocyte function within borderline lesions, therefore, offering valuable information regarding multi-step hepatocarcinogenesis. In this way, decreased uptake of Gd-EOB-DTPA may be an indicator of malignant change (34, 35). CE-MR imaging may also increase the conspicuity and delineation of tumor margins compared with CT, the visualization of intra-hepatic vessels, and provide a map of multinodular HCCs for planning subsegmental TACE. HBPI may also provide additional information for use in differential diagnoses (see next section).
Consensus statement: A hypointense nodule in the hepatobiliary phase of Gd-EOB-DTPA that is non-hypervascular in the arterial phase is regarded as intermediate risk whereas one that is hypervascular in the arterial phase is high-risk.
The discovery of liver masses during investigations for unrelated clinical problems is increasing, which may, in part, be due to the widespread use of advanced imaging techniques (36). As management of these incidental lesions is taking up increasing amounts of clinical practice it is important to gain a confident diagnosis early to reduce further investigation time and associated costs. There are many diagnostic algorithms for incidental lesions presented in the literature, but no existing consensus on the best sequence of modalities to use. Imaging must be based on the underlying clinical context, diagnostic accuracy, patient safety, and cost efficacy (37-39). MR imaging is one of the most accurate imaging modalities for differential diagnoses, allowing positive identification in 70% of cases (40), and is complimentary to other techniques such as CEUS (41). Since both vascularity and hepatobiliary uptake add important information on the differential diagnosis an algorithm for image interpretation based on these imaging findings (along with other findings from T2-weighted [T2W] sequences and diffusion-weighted imaging [DWI]) is considered to be helpful for image interpretation in daily practice. However, it should be kept in mind that the wide variety of all differential diagnoses cannot be covered in a simple algorithm and that atypical image manifestations can be seen from time to time.
Consensus statement: The proposed algorithm in Figure 6 for the work-up of incidental lesions using Gd-EOB-DTPA MR imaging in patients without increased risk of HCC is appropriate.
The range of lesions observed in the non-cirrhotic liver is larger than for cirrhotic organs, from benign masses such as focal fatty infiltration or focal nodular hyperplasia (FNH) to malignant lesions, including metastases, cholangiocarcinomas or HCCs (40). Older age (> 55 years), enlarged liver and a raised serum alkaline phosphatase are associated with malignancy, but there are no reliable clinical characteristics that can distinguish benign from malignant lesions (42). Malignant lesions can appear in patients who are otherwise healthy; 11 unexpected malignant lesions were found in 64 healthy patients followed-up for five years (42).
Certain radiographic features such as size, distribution, and morphology are helpful in distinguishing between benign and malignant, but can be unreliable for diagnosis. Multiple liver lesions may be indicative of metastases, but rare cases of multiple cystic masses, for example, can occur. Likewise, single large masses may be indicative of hemangioma, but some carcinoma may present a similar initial picture. T2W MR images provide better evidence for lesion type; signal intensity compared with surrounding parenchyma gives a good indication as to the threat of the mass. DWI is also an informative tool, with lesion signal changes between low and high b values being particularly important. However, this assessment is less reliable for the differential diagnosis within the group of solid lesions than for cystic masses or hemangiomas.
Whilst the vascularity and portal venous washout patterns of liver lesions remains an essential tool for diagnosis, in many cases the added information gained from hepatobiliary phase imaging is essential for an accurate diagnosis (as shown in Fig. 5), without the need for invasive sampling, especially for solid tumors. In this respect, the presence of regular hepatocellular uptake helps to classify liver lesions and, therefore, represents an important step for the characterization of lesions (43). For this reason, the evaluation of the signal intensity of an unclear liver mass in the hepatobiliary phase might be a valuable approach as shown in the proposed algorithm. If regular hepatocellular uptake is seen (in line with other typical imaging features), than a benign nature of a lesion is very likely.
Common lesions found in cirrhotic liver are hyperplastic nodules, DNs, FNH-like nodules, and HCCs, all of which tend to show hyperintensity in the hepatobiliary phase (44). DNs are usually small (< 1.5 cm diameter), homogeneous, isointense on T2W imaging, show no arterial hypervascularity, and are hyper- or isointense in the hepatobiliary phase. During follow up of these types of lesions, any arterial hypervascularity or reduction in hepatobiliary phase signal intensity (hypointensity) might suggest malignant change.
Focal nodular hyperplasia-like nodules are believed to arise as the result of a local hyperplastic response to decreased portal venous and increased hepatic arterial blood flow. They are not considered premalignant and have imaging features similar to classical FNH, but they are seen in patients with hepatic vascular abnormalities or chronic liver disease. In patients with cirrhosis, FNH-like nodules are often difficult to differentiate from well differentiated HCCs, due to the arterial phase enhancement, but unlike HCCs, they show hyperintensity in the hepatobiliary phase with a hypointense central, stellate-like scar. FNH-like nodules are also frequently multiple in occurrence and show minimal growth during long-term follow-up.
Around 5-10% of HCCs (grade 1-2) appear as hyperintense in the hepatobiliary phase of Gd-EOB-DTPA MR imaging. These can be differentiated correctly using features of HBPI, including heterogeneous uptake of contrast agent, a hypointense rim suggestive of a pseudocapsule, and internal septation due to differential histological components within the lesion. Mimickers of HCC in high-risk patients are bile duct adenoma (peribiliary hamartoma), small arterial-enhancing nodules, and cholangiocarcinoma. Bile duct adenoma is a benign, non-cystic, non-encapsulated, small (< 2 cm) lesion involving the ductules, inflammation and fibrosis. These lesions are thought to be reactive processes to focal injury, and may be associated with chronic liver disease (45-47). These show hypervascularity and delayed enhancement. Small arterially-enhancing lesions can be differentiated from small HCCs as they do not show hyperintensity on T2W images or a hypointense defect on HBPI. However, some very small arterially-enhancing lesions (< 1 cm) can show clear portal venous washout and hepatobiliary defect.
Cholangiocarcinoma is a malignant lesion, but should be differentiated from HCC as the treatment plans are different in many cases. Cholangiocarcinomas are homogeneous, with an irregular lobulated margin, and no capsule. The periphery usually contains arterially-enhancing viable tumor cells, with variable degrees of fibrosis and coagulative necrosis in the central lesion. These lesions tend to show irregular peripheral and gradual centripetal enhancement, with prominent delayed enhancement being common on ECCM MR imaging (which may not occur on Gd-EOB-DTPA MR imaging). Importantly, sclerosing hemangioma or tuberculosis in a hepatitis B virus patient can show a similar enhancement patterns to cholangiocarcinoma, and these lesions may require a histological confirmation for diagnosis. Solitary metastases may also be difficult to differentiate from cholangiocarcinoma, even on histologic examination. However, the presence of central necrosis, which is rare in cholangiocarcinoma, indicated by strong high signal intensity on T2W images, may favor the diagnosis of metastasis.
Consensus statement: In patients without risk of HCC, substantial hepatobiliary uptake indicates a benign lesion.
Other challenging diagnoses in the Asian population are inflammatory lesions (such as migrating, focal eosinophilic lesions) or unexpected HCC in otherwise healthy patients. Focal steatosis is an uncommon lesion type that may be encountered after a patient has undergone chemotherapy, and can appear similar to HCC with fatty changes.
Consensus statement: Gd-EOB-DTPA MR imaging is considered useful as the next step to characterize a liver lesion that is unclear on US or CE-CT.
Discussing patient diagnosis and subsequent treatment options within multidisciplinary teams is recognized as an optimal approach to patient management (48). A key aim of such discussions between surgeons, gastroenterologists, oncologists and radiologists is to identify candidates for curative interventions - i.e. not to deny treatment to patients with potentially curable disease by overstaging, or subject patients to futile procedures by understaging.
Using colorectal cancer liver metastases (CRCLM) as an example of pre-surgical management - evidence suggest that around 50% of patients with colorectal cancer have metastases in the liver at the time of diagnosis of the primary tumor. Approximately 50% of liver metastases will be detected synchronous to the primary tumor, while the rest will become detectable later, as metachronous disease (49, 50). Operative mortality and morbidity for CRCLM are low, resulting in a 5-year survival of 38-55% (49). Failure to achieve long-term survival is largely due to the high frequency of extra-hepatic recurrence (often in combination with intra-hepatic recurrences), which account for about two thirds of recurrences (51, 52). The remaining third of cases, however, have isolated intra-hepatic recurrence. Logically, in some of these patients, higher accuracy in the detection of all intra-hepatic disease could have resulted in long-term survival with modifications in the surgical approach. The limitations of currently available imaging modalities (US, CT, conventional MR imaging and fluorodeoxyglucose positron emission tomography [FDG-PET]), in terms of the per patient and per lesion sensitivity, especially for lesions in the sub-centimeter range, still presents a major problem (53, 54).
A large range of normal anatomical variations exist regarding both the biliary and vascular anatomy of the liver (55-58). A summary of the pre-surgery requirements and clinical motivation for the information is presented in Table 1. Prior to intervention, the surgeon requires information regarding the anatomy of the hepatic vascular supply and drainage and biliary tree, as well as proximity to, or engagement of these structures by tumor. Having a "road map" of the liver vasculature aids in a safer dissection and isolation of vascular structures in order to gain vascular control before commencing parenchymal transection. Prior knowledge of the vascular variants also decreases the risk for arterial and portal venous devascularization as well as venous stasis of the post-operative liver remnant. Whereas the hepatic parenchyma has a dual blood supply (75% coming from the portal vein and 25% from the hepatic artery), the supply of the biliary tree is exclusively arterial making it more vulnerable for arterial devascularization especially with damage to the more distal vasculature where the potential for formation of collaterals is limited. Congestion of the parenchyma in the remnant liver, bordering the resection line must be prevented by ensuring sufficient venous drainage. Not only is function in the congested liver decreased, but even the regenerative capacity is compromised (59, 60).
Consensus statement: CE-MR imaging is the most sensitive pre-operative imaging modality used in cirrhosis cases, such as HCC whereas MDCT (followed by CE-MR imaging) is the standard pre-operative imaging modality used in secondary liver lesions, such as colorectal cancer. Both MDCT and CE-MR imaging are used as standard pre-operative imaging modality in transplant cases.
Liver failure is the biggest cause of post-operative mortality after liver resection. Therefore, making an accurate pre-operative prediction of post-operative liver function and regeneration capacity is vital (61-63). In individuals with normal liver function undergoing liver resection, such as patients with CRCLM, volume-based decisions regarding resection are appropriate, with a remnant of 20-30% of the original volume usually being sufficient for maintaining post-operative function and for sufficient regeneration. In patients with parenchymal dysfunction, volume-based decision-making alone is obsolete. The worse the liver function, the bigger the volume needed for maintaining function and allowing sufficient regeneration. In patients with homogeneous disease a global liver function test is probably enough for the functional analysis. However, in the case of inhomogeneous liver disease, a liver function test that can quantify function on a segmental level is needed for accurate predictions. Previously, liver cirrhosis was the most commonly encountered parenchymal disease in liver resection patients. The increasing use of pre-operative neoadjuvant chemotherapy in resection candidates for CRCLM with ensuing chemotherapy-induced parenchymal damage and the metabolic syndrome-associated liver diseases (non-alcoholic fatty liver disease [NAFLD] and non-alcoholic steatohepatitis [NASH]) in the wake of the obesity epidemy add some new challenges (64).
Currently used liver function tests (e.g. analyte measurements and clearance tests) measure total liver function (65, 66). We know however, that liver function is not homogeneous in some chronic liver diseases such as primary sclerosing cholangitis, alcoholic cirrhosis, NAFLD and NASH. There is also some evidence that function is not equally distributed in the healthy liver (67-72).
Dynamic hepatocyte-specific contrast-enhanced MR imaging (DHCE-MR imaging) with Gd-EOB-DTPA is based on the principle of a dynamic liver function test using an imaging modality as sampling method (72). A number of quantitative functional parameters such as hepatic extraction fraction (HEF), area under the curve, input relative blood flow and mean transit time can be calculated on a segmental level enabling the calculation of a volume-function value for each segment. Total and residual liver functions can be calculated by adding these values, with the added values correcting for eventual regional variation in function. HEF, for example, describing the amount of Gd-EOB-DTPA which would be eliminated in one pass through the liver, is decreased in patients with primary biliary cirrhosis, with values decreasing in correlation with increasing grade of biliary cirrhosis (73).
MR imaging can provide high-level data regarding vascular and biliary complications following surgery. Some information, such as vessel configurations or crossing and susceptibility artifacts from cholecystectomy clips, can be discerned equally well on native T2W or CE-T1-weighted (T1W) scans. However, the extra dimension of time is added, using a hepatocyte-specific contrast agent, allowing the tracking of the contrast agent in the biliary tree (Fig. 7). That can be helpful for diagnosis of post-surgical biliary complications such as leakage and stenosis in bilio-biliary or bilio-enteric anastomosis. Likewise, vascular complications can be confidently diagnosed using MR imaging, including aneurysm formation, liver infarction or post-surgical bleeding.
Consensus statement: CE-MR imaging is the standard imaging modality used post-operatively to assess biliary complications.
Gd-EOB-DTPA-enhanced MR imaging has the potential to be a "one-stop shop" for evaluation of patients that are candidates for hepatic resection, in terms of diagnosis of the primary disease, surgical planning and diagnosis and assessment of post-operative complications. In an extended protocol, DHCE-MR imaging shows promise as a method for functional analysis with the possibility of segmental functional assessment that might be superior to currently available function tests in the prediction of post-operative liver function. It is envisaged that the consensus statements generated during the liver forum, based on evidence-based discussions, will improve diagnostic accuracy, patient outcomes and contribute to updates of clinical guidelines.
Figures and Tables
 | Fig. 1Image illustrating appearance of grade I foci in dysplastic nodule (A) and grade II foci in early hepatocellular carcinoma (B). |
 | Fig. 2Distribution of lesion type according to size. Most nodules may be sub-clinical (i.e. ≤ 5 mm) (15). DN = dysplastic nodule, eHCC = early hepatocellular carcinoma, G = grade, HGDN = high-grade dysplastic nodule, LGDN = low-grade dysplastic nodule |
 | Fig. 3Signal intensity on hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging and expression of OATP8 during multi-step hepatocarcinogenesis. eHCC = early hepatocellular carcinoma, HGDN = high-grade dysplastic nodule, LGDN = low-grade dysplastic nodule, OATP8 = organic anion-transporting polypeptide 8, RN = regenerative nodule |
 | Fig. 4Correlation between tumor enhancement ratio on hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging and amount of OATP8 expression at polymerase chain reaction. Expression score = (tumor transporter value/tumor b-actin value)/(background transporter value/background b-actin value). Enhancement ratio = (pre-enhancement signal intensity [SI] minus post-enhancement SI)/pre-enhancement SI. Reprinted, with permission, from Kitao et al. Radiology 2010;256(3):817-826. © Radiological Society of North America (7). |
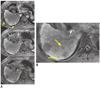 | Fig. 5Dynamic phase of Gd-EOB-DTPA MR imaging (A) shows clear arterial enhancement and washout of two hepatocellular carcinomas but hepatobiliary phase (B) also shows several subcentimeter hypointense nodules confirmed as well-differentiated, hepatocellular carcinoma. |
 | Fig. 6Proposed algorithm for work-up of incidental lesions using Gd-EOB-DTPA MR imaging in patients without increased risk of HCC. CE-CT = contrast enhanced CT, DWI = diffusion-weighted imaging, FNH = focal nodular hyperplasia, HBP = hepatobiliary phase, HCC = hepatocellular carcinoma, T1W = T1-weighted, T2W = T2-weighted, THID = transient hepatic signal intensity differences, US = ultrasound |
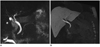 | Fig. 7Case study of 50-year-old male, post-cholecystectomy, with mild, common bile duct dilation.
T2-weighted MR imaging data (A) might suggest stenosis in pre-papillary area, but in corresponding Gd-EOB-DTPA-enhanced T1-weighted MR imaging (B) contrast agent was excreted into duodenum after 15 minutes, ruling out possibility of stenosis.
|
References
1. Liver Cancer Incidence and Mortality Worldwide in 2008 Summary. 2010. Last accessed December 13. Available at: http://globocan.iarc.fr/factsheets/cancers/liver.asp.
2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
3. Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004. 14:559–578.
4. Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005. 40:715–724.
5. Shuter B, Tofts PS, Wang SC, Pope JM. The relaxivity of Gd-EOB-DTPA and Gd-DTPA in liver and kidney of the Wistar rat. Magn Reson Imaging. 1996. 14:243–253.
6. Vander Elst L, Maton F, Laurent S, Seghi F, Chapelle F, Muller RN. A multinuclear MR study of Gd-EOB-DTPA: comprehensive preclinical characterization of an organ specific MRI contrast agent. Magn Reson Med. 1997. 38:604–614.
7. Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010. 256:817–826.
8. Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009. 44:793–798.
9. Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007. 17:2519–2530.
10. International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009. 49:658–664.
11. Kojiro M, Nakashima O. Histopathologic evaluation of hepatocellular carcinoma with special reference to small early stage tumors. Semin Liver Dis. 1999. 19:287–296.
12. Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998. 28:1241–1246.
13. Sakamoto M, Effendi K, Masugi Y. Molecular diagnosis of multistage hepatocarcinogenesis. Jpn J Clin Oncol. 2010. 40:891–896.
14. Oikawa T, Ojima H, Yamasaki S, Takayama T, Hirohashi S, Sakamoto M. Multistep and multicentric development of hepatocellular carcinoma: histological analysis of 980 resected nodules. J Hepatol. 2005. 42:225–229.
15. Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991. 22:172–178.
16. Sakamoto M, Hirohashi S. Natural history and prognosis of adenomatous hyperplasia and early hepatocellular carcinoma: multi-institutional analysis of 53 nodules followed up for more than 6 months and 141 patients with single early hepatocellular carcinoma treated by surgical resection or percutaneous ethanol injection. Jpn J Clin Oncol. 1998. 28:604–608.
17. Kudo M. The 2008 Okuda lecture: Management of hepatocellular carcinoma: from surveillance to molecular targeted therapy. J Gastroenterol Hepatol. 2010. 25:439–452.
18. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003. 33:Suppl 2. 1–5.
19. Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000. 275:23161–23168.
20. Vavricka SR, Jung D, Fried M, Grutzner U, Meier PJ, Kullak-Ublick GA. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. J Hepatol. 2004. 40:212–218.
21. Tsuda N, Matsui O. Cirrhotic rat liver: reference to transporter activity and morphologic changes in bile canaliculi--gadoxetic acid-enhanced MR imaging. Radiology. 2010. 256:767–773.
22. Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010. 59:638–644.
23. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.
24. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011. 53:1020–1022.
25. Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society of Hepatology 2009 update. Hepatol Res. 2010. 40:Suppl 1. 2–144.
26. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008. 134:1752–1763.
27. Lee JM, Kim SJ, Kim SH, Kim KW, Lee JY, Han JK, et al. Enhancement patterns of hepatocellular carcinoma on gadoxetic acid-enhanced MR imaging in the cirrhotic liver: comparison with multiphasic liver CT. In : RSNA 2009; Abstract no. SSA07-07.
28. Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010. 255:459–466.
29. Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010. 256:806–816.
30. Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010. 45:133–141.
31. Marin D, Di Martino M, Guerrisi A, De Filippis G, Rossi M, Ginanni Corradini S, et al. Hepatocellular carcinoma in patients with cirrhosis: qualitative comparison of gadobenate dimeglumine-enhanced MR imaging and multiphasic 64-section CT. Radiology. 2009. 251:85–95.
32. Sun HY, Lee JM, Shin CI, Lee DH, Moon SK, Kim KW, et al. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (< or = 2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010. 45:96–103.
33. Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Muhi A, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology. 2010. 256:151–158.
34. Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol. 2002. 178:885–897.
35. Kogita S, Imai Y, Okada M, Kim T, Onishi H, Takamura M, et al. Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol. 2010. 20:2405–2413.
36. Boutros C, Katz SC, Espat NJ. Management of an incidental liver mass. Surg Clin North Am. 2010. 90:699–718.
37. Marin D, Furlan A, Federle MP, Midiri M, Brancatelli G. Imaging approach for evaluation of focal liver lesions. Clin Gastroenterol Hepatol. 2009. 7:624–634.
38. Chamberlain RS, Jarnagin WR, DeCorato D. Steele GD, Phillips TL, Chabner BA, editors. Incidentally discovered hepatic lesions. Hepatobiliary Cancer. 2001. Hamilton, London: American Cancer Society Atlas of Clinical Oncology;31–42.
39. Zech CJ, Grazioli L, Jonas E, Ekman M, Niebecker R, Gschwend S, et al. Health-economic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast media-enhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur Radiol. 2009. 19:Suppl 3. S753–S763.
40. Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009. 15:3217–3227.
41. Soussan M, Aube C, Bahrami S, Boursier J, Valla DC, Vilgrain V. Incidental focal solid liver lesions: diagnostic performance of contrast-enhanced ultrasound and MR imaging. Eur Radiol. 2010. 20:1715–1725.
42. Little JM, Richardson A, Tait N. Hepatic dystychoma: a five year experience. HPB Surg. 1991. 4:291–297.
43. Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO. Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol. 2008. 43:504–511.
44. Kamaya A, Maturen KE, Tye GA, Liu YI, Parti NN, Desser TS. Hypervascular liver lesions. Semin Ultrasound CT MR. 2009. 30:387–407.
45. Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988. 12:708–715.
46. Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996. 20:858–864.
47. Tajima T, Honda H, Kuroiwa T, Yoshimitsu K, Irie H, Aibe H, et al. Radiologic features of intrahepatic bile duct adenoma: a look at the surface of the liver. J Comput Assist Tomogr. 1999. 23:690–695.
48. Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10-year study of outcome following hepatic resection for colorectal liver metastases - The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol. 2009. 35:302–306.
49. Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007. 11:1057–1077.
50. Nordlinger B, Van Cutsem E, Rougier P, Kohne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007. 43:2037–2045.
51. Ahmad A, Chen SL, Bilchik AJ. Role of repeated hepatectomy in the multimodal treatment of hepatic colorectal metastases. Arch Surg. 2007. 142:526–531. discussion 531-522.
52. Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007. 14:1151–1160.
53. Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002. 224:748–756.
54. Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology. 2005. 237:123–131.
55. Marcos A, Ham JM, Fisher RA, Olzinski AT, Posner MP. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000. 231:824–831.
56. Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008. 28:359–378.
57. Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg. 2000. 7:580–586.
58. Uchida K, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Ota M, et al. Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation. Liver Transpl. 2010. 16:1062–1068.
59. Scatton O, Plasse M, Dondero F, Vilgrain V, Sauvanet A, Belghiti J. Impact of localized congestion related to venous deprivation after hepatectomy. Surgery. 2008. 143:483–489.
60. Radtke A, Sotiropoulos GC, Sgourakis G, Molmenti EP, Schroeder T, Saner FH, et al. Hepatic venous drainage: how much can we learn from imaging studies? Anatomic-functional classification derived from three-dimensional computed tomography reconstructions. Transplantation. 2010. 89:1518–1525.
61. Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000. 191:38–46.
62. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002. 236:397–406. discussion 406-407.
63. Clavien PA, Oberkofler CE, Raptis DA, Lehmann K, Rickenbacher A, El-Badry AM. What is critical for liver surgery and partial liver transplantation: size or quality? Hepatology. 2010. 52:715–729.
64. Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010. 17:457–463.
65. Gazzaniga GM, Cappato S, Belli FE, Bagarolo C, Filauro M. Assessment of hepatic reserve for the indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005. 12:27–30.
66. Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009. 13:374–385.
67. Jonas E, Hultcrantz R, Slezak P, Blomqvist L, Schnell PO, Jacobsson H. Dynamic 99Tcm-HIDA SPET: non-invasive measuring of intrahepatic bile flow. Description of the method and a study in primary sclerosing cholangitis. Nucl Med Commun. 2001. 22:127–134.
68. Harbin WP, Robert NJ, Ferrucci JT Jr. Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology. 1980. 135:273–283.
69. Garcia JE, Atkins F. A low right-to-left hepatic lobe ratio. Is streamlining of ethanol to the right lobe of the liver the cause? Clin Nucl Med. 1985. 10:807–809.
70. Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006. 44:874–880.
71. Nilsson H, Nordell A, Vargas R, Douglas L, Jonas E, Blomqvist L. Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J Magn Reson Imaging. 2009. 29:1323–1331.
72. Jacobsson H, Jonas E, Hellstrom PM, Larsson SA. Different concentrations of various radiopharmaceuticals in the two main liver lobes: a preliminary study in clinical patients. J Gastroenterol. 2005. 40:733–738.
73. Nilsson H, Blomqvist L, Douglas L, Nordell A, Jonas E. Assessment of liver function in primary biliary cirrhosis using Gd-EOB-DTPA-enhanced liver MRI. HPB (Oxford). 2010. 12:567–576.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download