Abstract
We present two cases of individual pulmonary vein atresia without vestige of an involved pulmonary vein. On CT, we noted the absence or interruption of normal pulmonary venous structures, and the presence of abnormal vascular structures that represented collaterals for the involved lung parenchyma. On angiography, the atretic pulmonary vein was found to drain into the other ipsilateral pulmonary veins through the collaterals.
Pulmonary vein stenosis and atresia may occur in one or more vessels and may present as an isolated anomaly or be associated with other congenital cardiac anomalies (1-3). Pulmonary vein atresia can be divided into 3 categories according to the extent of pulmonary vein involvement: common, unilateral and individual pulmonary vein atresia. Common and unilateral pulmonary vein atresias have been reported in infants and children, but rarely occur in adults.
Individual pulmonary vein atresia is defined as atresia of the lobar or segmental pulmonary vein and involves less than the unilateral lung. Only a few cases of individual pulmonary vein atresia in infants and young children have been reported (2). Severe individual pulmonary vein stenosis was previously observed in a 28-year-old man in whom a markedly narrowed ostium of the inferior pulmonary vein near the left atrium and appeared as a thin string-like vestige structure on CT (4).
To the best of our knowledge, the CT and angiographic findings of individual pulmonary vein atresia without a vestige of the involved pulmonary vein have not been reported. In this article, we present the imaging findings of individual pulmonary vein atresia in two adults along with a review of the relevant literature.
A 32-year-old man who had no upper respiratory symptoms or hemoptysis was admitted to the hospital for a surgical procedure to correct a herniated intervertebral disc. He underwent a chest radiograph for a preoperative evaluation.
The chest radiograph and the chest CT revealed evidence of pulmonary tuberculosis in both upper lobes. The CT scan revealed an interrupted right inferior pulmonary vein with a low-attenuation linear structure at the venoatrial junction. Also evident were abnormally enlarged vascular structures near the right pulmonary artery (Fig. 1A-D), which indicate the presence of collaterals between the superior pulmonary vein and the interrupted inferior pulmonary vein (Fig. 1A).
Pulmonary angiography revealed that the interrupted right inferior pulmonary vein drained into the superior pulmonary vein through communicating veins. Based on these findings, the diagnosis was individual pulmonary vein atresia of the right lower lobe (Fig. 1E).
Echocardiography demonstrated only mild pulmonary hypertension with no evidence of cardiac defect or cardiomegaly. Since there was no evidence of a congenital anomaly, we decided on the conservative treatment route accompanied by close follow-up at the outpatient department.
A 43-year-old man presented with persistent cough, sputum and no episode of hemoptysis. A chest radiography revealed a nodular density measuring 8 mm and located in the right upper lobe. In addition, we noted an abnormal tubular communicating vascular structure as well as tortuous or dot-like collaterals in the anterior segment of the right upper lobe on the CT scan. The right superior pulmonary vein was absent in the normal position (Fig. 2A, B).
The patient underwent a pulmonary angiography for coil embolization of the suspected arteriovenous malformation. Selective injection of contrast material into the apical segment of the right upper lobe demonstrated that the apical segmental pulmonary vein was interrupted and drained into other segmental pulmonary veins through collateral veins (Fig. 2C).
We diagnosed these findings as individual pulmonary vein atresia of the apical segment in the right upper lobe. We could not evaluate for a cardiac defect because the patient refused to undergo an echocardiography. The electrocardiogram showed only sinus bradycardia and no evidence of right ventricular hypertrophy. Since the patient's symptoms were mild, we did not take any measures aside from a regular follow-up at the outpatient clinic were taken.
Pulmonary vein atresia can be divided into 3 categories: common, unilateral, and individual pulmonary vein atresia. These categories are based on the extent of involvement and this depends on the stage in which the normal development of the pulmonary venous drainage is affected (5). Individual pulmonary vein atresia is defined as atresia of the lobar or segmental pulmonary vein which involves symptoms less significant than unilateral pulmonary vein atresia. Pulmonary vein atresia can also be divided into congenital and acquired atresia. Congenital pulmonary vein atresia appears to occur from defective incorporation of the common pulmonary trunks into the left atrium, which leads to the obstruction of some or all of its branches. In rare cases of unilateral and individual pulmonary vein atresia, the venous obstruction may be acquired secondary to veno-occlusive disease or mediastinitis (2, 5).
In common pulmonary vein atresia, the pulmonary veins do not connect to the left atrium. Instead, they join together into a venous confluence close to the posterior aspect of the left atrium and drain into the bronchial venous system or the esophageal vein. An obligatory right-to-left shunt is present at the atrial level. Atresia of the common pulmonary vein is a cyanotic condition. The prominent symptoms include marked cyanosis, tachypnea, and dyspnea.
Unilateral pulmonary vein atresia may occur on either side of the lung with no right- or left-side predominance. The most frequent presenting complaints for infant cases include recurrent infections in the hypoplastic lung and hemoptysis due to the systemic collateral supply to the affected lung (6, 7). Unilateral pulmonary vein atresia can be identified in isolation or it may be found in patients with more severe congenital heart malformations. The involved lung becomes progressively smaller, and even the contralateral lung may appear edematous, reflecting the maldistribution of blood flow. In most patients, unilateral pulmonary vein atresia is diagnosed during infancy and only a few adult cases have been reported (1, 6).
In this article, individual pulmonary vein atresia refers to complete obliteration of an individual pulmonary vein in isolation. In contrast to atresia of the individual pulmonary vein, individual pulmonary vein stenosis can be defined as narrowing of an individual pulmonary vein. When the severe stenotic process is aggravated, it may manifest as atresia of a pulmonary vein. However, in cases of individual pulmonary vein stenosis, opacification of the stenotic pulmonary vein can be obtained by selective right and left pulmonary angiography.
Only a few cases of individual pulmonary vein atresia in infants and young children have been reported. Cullen et al. (2) reported on two children with individual pulmonary vein atresia and they were without additional congenital heart disease (2, 3). Both patients presented with recurrent pulmonary infections or hemoptysis. The radiographic findings showed a hypoplastic pulmonary artery ipsilateral to the pulmonary vein atresia. Venous drainage from the affected lung occurred into the systemic venous system via the azygos vein and bronchial venous system. Individual pulmonary vein atresia is a very rare condition; especially in adults. One adult case of severe stenosis of an individual pulmonary vein was reported (8). The findings of the report indicated a markedly narrowed ostium of the inferior pulmonary vein near the left atrium, which appeared as a thin string-like vestige structure on CT. Angiography demonstrated a tortuous vessel ascending the cephalad during the delayed phase as a collateral pathway (4).
In our study, the first case was lobar pulmonary vein atresia of the right lower lobe, whereas the second case was segmental pulmonary vein atresia of the apical segment located in the right upper lobe. For the first case, the right inferior pulmonary vein was atretic in the venoatrial junction, and drained into the superior pulmonary vein through the collaterals. For the second case, the apical segmental pulmonary vein of the right upper lobe was interrupted and it drained into another segmental pulmonary vein of the right upper lobe through the collaterals. On both cases, the ipsilateral main pulmonary artery was not hypoplastic. Both patients didn't show any symptoms such as hemoptysis, recurrent infection, or cardiac problems, which contrasts with the cases of infants and young children reported upon by Cullen et al. (2). Compared with the case of severe pulmonary stenosis reported by Saida et al. (4), there was no vestige of the affected pulmonary vein in our first case.
In summary, individual pulmonary vein atresia rarely occurs in adults without specific symptoms. The CT findings in our cases indicate the absence or interruption of normal pulmonary venous structures, and the presence of abnormal vascular structures that represented collaterals which developed for the involved lung parenchyma. Individual pulmonary atresia was confirmed by angiography in which the interrupted pulmonary vein drained into other ipsilateral pulmonary veins through the collaterals.
Figures and Tables
Fig. 1
Lobar pulmonary vein atresia of right lower lobe in 32-year-old man.
A. Serial axial images show low-attenuation linear structure (arrows) between right inferior pulmonary vein and left atrium, as well as prominent right superior pulmonary vein(s). Right pulmonary artery is not hypoplastic compared with left pulmonary artery. Also note abnormally enlarged vascular structure (double arrows) near right pulmonary artery, suggesting presence of collateral between superior pulmonary vein and interrupted inferior pulmonary vein on angiogram. B. Reformatted oblique sagittal image shows atretic right inferior pulmonary vein (black arrow). C. Serial axial images with lung setting show multiple dot-like collaterals between superior pulmonary vein and atretic inferior pulmonary vein in right lower lobe. D. Reformatted coronal image shows tortuous and dot-like collaterals (black arrowheads) in superior segment of right lower lobe. E. Angiogram of right lower lobe shows atretic right inferior pulmonary vein (double arrowheads) draining into superior pulmonary vein (arrowhead) through collaterals.
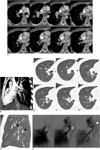
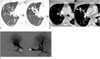
Fig. 2
Segmental pulmonary vein atresia of apical segment from right upper lobe in 43-year-old man.
A. Slap axial images with lung setting show tortuous and dot-like collaterals in anterior segment of right upper lobe. In addition, abnormal tubular vascular structure (arrowhead) is present in anterior segment of right upper lobe. This finding is consistent with imaging findings of pulmonary arteriovenous malformation, but we diagnosed it as collateral, from apical segment to anterior segment of right upper lobe on pulmonary angiography. B. Serial axial images show absence of right superior pulmonary vein in normal position and presence of collaterals (arrowhead) draining into segmental pulmonary vein of right upper lobe. C. Angiogram of apical segment of right upper lobe shows that apical segmental pulmonary vein is interrupted (arrow) and drains into another segmental pulmonary vein through collaterals (arrowhead).
References
1. Heyneman LE, Nolan RL, Harrison JK, McAdams HP. Congenital unilateral pulmonary vein atresia: radiologic findings in three adult patients. AJR Am J Roentgenol. 2001. 177:681–685.
2. Cullen S, Deasy PF, Tempany E, Duff DF. Isolated pulmonary vein atresia. Br Heart J. 1990. 63:350–354.
3. Freedom RM, Mawson JB, Yoo S, Benson LN. Congenital heart disease: textbook of angiocardiography. vol. II. 1997. New York: Futura Publishing;665–691.
4. Saida Y, Eguchi N, Mori K, Tanaka YO, Ishikawa S, Itai Y. Isolated pulmonary vein stenosis associated with full intrapulmonary compensation. AJR Am J Roentgenol. 1999. 173:961–962.
5. Pourmoghadam KK, Moore JW, Khan M, Geary EM, Madan N, Wolfson BJ, et al. Congenital unilateral pulmonary venous atresia: definitive diagnosis and treatment. Pediatr Cardiol. 2003. 24:73–79.
6. Swischuk LE, L'Heureux P. Unilateral pulmonary vein atresia. AJR Am J Roentgenol. 1980. 135:667–672.
7. Kuhn M. Cardiac and intestinal natriuretic peptides: insights from genetically modified mice. Peptides. 2005. 26:1078–1085.
8. Amplatz K, Moller JH. Radiology of congenital heart disease. 1993. St. Louis: Mosby;847–909.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download