Abstract
We report a case of thymic hyperplasia accompanied by pericardial lipomatosis and right facial hemihypertrophy in an 8-year-old boy. On imaging studies, the hyperplastic thymus had prominent curvilinear and nodular fatty areas simulating a fat-containing anterior mediastinal mass, which is an unusual finding in children. To our knowledge, this is the first report on a child with a combination of thymic hyperplasia, pericardial lipomatosis, and right facial hemihypertrophy. The radiologic findings are presented with a brief discussion.
Thymic hyperplasia is defined as the enlargement of the thymus with a histologically normal cortical and medullary component. The hyperplastic gland can become very large; however, besides its larger size, the imaging appearance of the enlarged thymus is normal (1). No child cases of pericardial lipomatosis have been reported in the English literature. To the best of our knowledge, this is the first case report of a child with thymic hyperplasia simulating a fat containing mass accompanied by pericardial lipomatosis and right facial hemihypertrophy.
An 8-year-old boy visited the outpatient clinic for evaluation of facial asymmetry and narrowing of the right external auditory canal. Upon physical examination, the patient showed right side facial enlargement, cranial protrusion, gingival hypertrophy, and soft tissue thickening of the external auditory canal. There was no evidence of asymmetry of the body trunk and limbs or any subcutaneous/cutaneous lesions noted. The patient denied any prior history of medical, surgical problems, or any family history of hemihyperplasia. A chest posteroanterior film identified a large mediastinal mass draping over the right cardiac shadow (Fig. 1A). A chest lateral image indicated a mass filling the anterior mediastinal space (Fig. 1B). For further evaluation, contrast-enhanced chest CT was performed. Chest CT revealed a large mass occupying the anterior mediastinum showing no mass effect or displacement of adjacent structures. It had a bilobular configuration with asymmetric mass-like enlargement of the right lobe which contained curvilinear or nodular areas of fatty component. The left lobe was slightly enlarged and showed relatively homogeneous attenuation. Concomitant diffuse fat attenuation filling the pericardial space suggestive of pericardial lipomatosis was also noted. No evidence of associated mediastinal lymphadenopathy or focal lung lesion was noted (Fig. 1C-F). A fat containing benign mediastinal mass including thymolipoma or lipoblastoma was suspected. A malignant mass such as liposarcoma or malignant germ cell tumor was also included in the differential diagnoses. As it was difficult to determine whether the more predominantly enlarged right lobe was the only portion involved or both the right and left lobes were pathologic, mediastinal MRI was performed to further determine the precise extent of the lesion. The MRI results revealed a large anterior mediastinal mass that showed heterogeneous iso-signal intensity to the chest wall muscles on T1- and T2-weighted images and heterogeneous enhancement on fat saturated T1-weighted images. There were whorls or nodular areas of high signal intensity within the thymus on both T1- and T2-weighted images which showed suppression on fat saturated contrast enhanced T1-weighted sequence. The pericardial space was also filled with high signal intensity on both T1- and T2-weighted images and showed fat suppression on fat saturated contrast enhanced T1-wieghted images (Fig. 1G, H). For evaluation of facial asymmetry and narrowing of the right external auditory canal, contrast-enhanced CT of the paranasal sinuses was also performed. Paranasal sinus CT showed hypertrophy of the right side parotid gland, adenoid tissue, temporalis muscle, and sternocleidomastoid muscle. The right inferior nasal turbinate was also prominent. Moreover, mild narrowing of the right external auditory canal was noted due to soft tissue thickening along the posterior wall of the canal (Fig. 1I-K).
The patient underwent surgery consisting of a total thymectomy with mediastinal lymph node dissection. The operative findings revealed a large anterior mediastinal mass showing no invasion into the mediastinal structures and the parietal pleura. On gross specimen, the mass was yellowish with a lobulating contour confined within a thin fibrous capsule. The mass was about 17 × 13 × 3 cm in size and weighed 280 g (Fig. 1L). The cut surface showed multifocal areas of light-yellow spots of fatty tissue scattered within the thymic mass. There was no evidence of hemorrhage or areas of cystic, necrotic changes (Fig. 1L). Microscopically, the tumor showed normal distribution of the cortex, medulla and Hassall's corpuscles. It had fibrous and myxoid stroma with focal areas of adipose tissue admixed in various proportions (Fig. 1M). The histologic diagnosis was a true thymic hyperplasia. The postoperative course was uneventful and the patient was discharged to follow-up on an outpatient basis for further management of right facial hemihypertrophy. The patient was followed up for about a year and a half and has not shown any evidence of progressive course or cutaneous/subcutaneous lesions thus far.
Thymic hyperplasia is defined as an enlarged thymus beyond the normal upper limit for any given patient age. Hence, the imaging appearance is similar to a normal thymus except for its larger size (1). Moreover, thymic hyperplasia shows homogeneous attenuation similar or slightly lower than the muscles on noncontrast CT and homogeneous enhancement of 20-30 Hounsfield units after contrast enhancement. On MRI, thymic hyperplasia show slightly brighter SI than that of the muscles on both T1-weighted and T2-weighted images (1-3). Opposed-phase images may show diffuse decreased signal intensity (4). A normal thymus has a homogeneous appearance in childhood and begins to appear heterogeneous on imaging studies at about the age of thirty when the adipocytes become dominant. Hence, any condition in which the thymus appears heterogeneous in a child should raise suspicion of a pathologic condition (3). In our case, the enlarged thymus had a heterogeneous appearance with areas of fat admixed within the thymic tissue. It was an unusual finding for a child and a fat containing anterior mediastinal mass such as thymolipoma, lipoblastoma was to be considered. Malignant lesions such as malignant germ cell tumor or liposarcoma was also considered as a possible differential diagnosis. The mass was confirmed to be a hyperplastic thymus composed of normal thymic components and cellular organization suggestive of a true thymic hyperplasia on histologic examination. Although it is well known that the thymus can undergo hyperplastic changes after suppression from recent stressful conditions such as chemotherapy for neoplasm, Grave's disease, corticosteroid therapy for Cushing's disease, irradiation or thermal burns and some autoimmune diseases (Systemic lupus erythematosus, Hashimoto thyroiditis, Addison's disease, acromegaly), our patient did not have any of these well known systemic stresses or conditions causing thymic hyperplasia (5). However, the patient did have facial hemi-hypertrophy on the right side. As it has been reported that the left lobe of the thymus is usually larger than the right, the predominant enlargement of the right lobe with more prominent fatty component on the right side shown in our case may be related to the underlying right side hypertrophic condition of this patient (2, 3).
Complimentary to this finding, the patient also had diffuse pericardial lipomatosis, which is also an extremely rare finding with only a few cases reported in adult patients (6, 7). Pericardial lipomatosis, whether isolated or accompanied by other diseases, has not been reported in the pediatric population. The combination of thymic hyperplasia, pericardial lipomatosis, and facial hemihypertrophy seen in our case is also the first to be reported, both in children and adults. Although the patient did not undergo any genetic studies, considering that the patient has facial hemihypertrophy, thymic hyperplasia, and deep soft tissue lipomatosis, there is a possibility that the clinical and imaging features of this patient could be a spectrum of hemihyperplasia syndromes. Hyperplasia syndromes include a heterogeneous group of disorders with overgrowth of one or more limbs or body parts as a predominant finding and includes disease entities such as Beckwith-Wiedemann syndrome, Proteus syndrome, Hemihyperplasia multiple lipomatosis syndrome (HHML), Cowden/Bannayan-Riley-Ruvalcaba syndrome (BRRS) among others. Beckwith-Wiedemann syndrome is the most well known hemihyperplasia syndrome and is associated with anomalies such as macroglossia, abdominal wall defects, hypoglycemia, and increased risk of embryonal tumors. It is also well known because of its genotypic abnormalities of the distal region of chromosome arm 11p. Proteus syndrome is a rare, highly variable hamartomatous syndrome which is clinically diagnosed if the patient satisfies the necessary diagnostic criteria of disproportionate overgrowth, connective tissue nevi, dysregulated adipose tissue, and vascular malformation. The gigantism of limbs or digits is pathognomonic and shows progressive enlargement. Cutaneous lesions such as connective tissue nevi, tends to appear over time and may delay the diagnosis. Some report patient with Proteus syndrome showing mutation of PTEN (Phosphatase and tensin homologue deleted on chromosome 10) and glypican 3 (GPC3)gene, while others cast doubts over its involvement. Hence the molecular role in the diagnosis of Proteus syndrome is controversial (8). HHML syndrome shows moderate abnormalities of hemihyperplasia with multiple lipomas. HHML syndrome is thought to be a distinct form of Proteus syndrome which does not satisfy the diagnostic criteria and shows less severe hyperplasia of the limbs or body parts. Currently, there are no molecular tests available for the diagnosis of HHML syndrome. BRRS and Cowden syndrome are caused by mutations in the PTEN gene. BRRS is characterized by macrocephaly, mild mental retardation, cutaneous lipomas, or hemangiomas, while pigmented macules of the glans penis and the onset is noted at birth or shortly thereafter. Cowden syndrome is an adult-onset condition with mucocutaneous signs and increased risk of cancer (9, 10). Among the disease entities of the hemihyperplasia syndromes listed above, the latter three are those which are known to show both hyperplasia and lipomas as in our case. However, as the asymmetric overgrowth in our patient was not from birth and the patient did not show any sign of mental retardation, BRRS may be excluded and the possible diagnosis could be narrowed down to Proteus syndrome or HHML syndrome. Until now, our patient was followed up for about a year and a half and the most fitting diagnosis appears to be HHML. However, although the patient did not show cutaneous, epidermal nevi or capillary, venous, and lymphatic malformation, they may develop over time and show progressive enlargement. Hence, further clinical observation and imaging studies including a serial skeletal survey will be necessary for the diagnosis or exclusion of Proteus syndrome. Unfortunately, genetic studies that may support the diagnosis of hemihyperplasia syndrome was not performed. Although the diagnosis and exclusion of several hyperplastic syndromes may be available with genetic studies, it may not be of any help in differentiating Proteus syndrome from HHML since there are no molecular studies available at this moment. Until molecular mechanisms of the hemihyperplasia syndromes are further elucidated, thorough observation of any change in the clinical findings will remain essential for the diagnosis of patients.
In conclusion, we have reviewed and discussed the imaging findings of a rare combination of thymic hyperplasia, pericardial lipomatosis and facial hemihypertrophy in a pediatric patient.
Figures and Tables
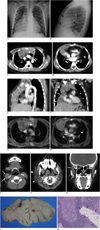
Fig. 1
8-year-old boy who had right facial hemihypertrophy, thymic hyperplasia and pericardial lipomatosis.
A. Chest posteroanterior film shows mass-like shadow (black arrows) draping around and obliterating right cardiac border extending towards costophrenic angle. B. On chest lateral image, mass-like shadow is filling anterior mediastinal space (double arrows). C, D. On contrast-enhanced chest CT, axial image reveals large thymic mass showing predominant enlargement of right lobe with no definite mass effect on adjacent structures. Mass shows heterogeneous iso-attenuation to that of chest wall muscles containing areas of curvilinear (black arrows) and nodular (open arrows) fat density foci which was also more prominent on right lobe (C). Diffuse homogeneous soft tissue lesion with fat attenuation is filling pericardial space (arrowheads), suggestive of pericardial lipomatosis (D). E, F. Predominant enlargement of right thymic lobe (white arrows) and pericardial lipomatosis (arrowheads) is also well demonstrated on reconstructed oblique sagittal (E) and coronal images (F) of contrast-enhanced chest CT. G, H. Mediastinal MRI with contrast-enhanced fat-saturated T1-weighted image shows suppression of curvilinear (black arrows) or nodular (open arrows) areas within thymic mass and pericardial space (arrowheads), suggestive of areas of fatty component. I-K. On contrast-enhanced paranasal sinus (PNS) CT, thickening of right sternocleidomastoid muscle (arrows) (I) is noted on axial image. Right parotid gland (arrowheads) and right adenoid (arrows) is also enlarged (J). Coronal image reveals thickening of right temporalis muscle (arrows) (K). L. On gross specimen, cut surface of thymic mass is soft and yellow with no evidence of cystic change, necrosis or hemorrhage. Light-yellow spots (arrows) of multifocal fatty tissue are scattered within mass. M. On microscopic examination, mass shows histologic composition of normal thymus composed of cortical and medullary components containing islands of fatty tissue (arrows) (Hematoxylin & Eosin staining × 100).
References
1. Thomas LS. The mediastinum, Caffey's pediatric diagnostic imaging. 2007. 11th ed. Philadelphia: Mosby Elsevier;1339–1344.
2. Baron RL, Lee JK, Sagel SS, Peterson RR. Computed tomography of the normal thymus. Radiology. 1982. 142:121–125.
3. Siegel MJ, Glazer HS, Wiener JI, Molina PL. Normal and abnormal thymus in childhood: MR imaging. Radiology. 1989. 172:367–371.
4. Takahashi K, Inaoka T, Murakami N, Hirota H, Iwata K, Nagasawa K, et al. Characterization of the normal and hyperplastic thymus on chemical-shift MR imaging. AJR Am J Roentgenol. 2003. 180:1265–1269.
5. Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The thymus: a comprehensive review. Radiographics. 2006. 26:335–348.
6. Akhtar A, D'Cruz IA, Ramanathan KB, Umpierrez M. Diffuse locally invasive lipomatosis of the pericardium. Echocardiography. 2003. 20:173–177.
7. Sanal HT, Kocaoglu M, Yildirim D, Ors F. Multiple cardiac lipomas and pericardial lipomatosis: multidetector-row computer tomography findings. Int J Cardiovasc Imaging. 2007. 23:655–658.
8. Thiffault I, Schwartz CE, Der Kaloustian V, Foulkes WD. Mutation analysis of the tumor suppressor PTEN and the glypican 3 (GPC3) gene in patients diagnosed with Proteus syndrome. Am J Med Genet A. 2004. 130A:123–127.
9. Dalal AB, Phadke SR, Pradhan M, Sharda S. Hemihyperplasia syndromes. Indian J Pediatr. 2006. 73:609–615.
10. Boybeyi O, Alanay Y, Kayikcioglu A, Karnak I. Hemihyperplasia-multiple lipomatosis syndrome: an underdiagnosed entity in children with asymmetric overgrowth. J Pediatr Surg. 2010. 45:E19–E23.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download