Abstract
Objective
The purpose of this study was to investigate retrospectively the clinical procedural performance of CT-guided needle biopsy for retroperitoneal lesions.
Materials and Methods
CT-guided needle biopsy was performed in 74 consecutive patients (M:F = 44:30; mean age, 59.7 years) with retroperitoneal lesions between April 1998 and June 2009. The target lesion ranged from 1.5 to 12.5 cm in size. The biopsy access path ranged from 3.5 to 11.5 cm in depth. A biopsy specimen was obtained using an 18-gauge core needle under a CT or CT-fluoroscopy guidance and with the patient under local anesthesia. The histopathological diagnoses from the biopsies were obtained. The diagnostic confirmation of the subtype of lymphoma was evaluated.
Results
Satisfactory biopsy samples were obtained in 73 (99%) of 74 patients and a pathological diagnosis was made in 70 (95%) of 74 patients. Sixty three lesions were malignant (45 lymphomas, nine primary tumors, nine lymph node metastases) and seven were benign. The subtype of lymphoma was specified in 43 (96%) of 45 patients who were diagnosed with lymphoma. Analysis of the value of CT-guided biopsy in this series indicated 63 true positives, zero false positive, six true negatives and five false negatives. This test had a sensitivity of 93%, a specificity of 100% and an accuracy of 93%. No major complications were seen and minor complications were noted in seven patients (five with local hematomas, two with transient pain at the puncture site).
It is necessary to obtain a histological sample of undiagnosed lesions for the planning of further therapies. Surgical or percutaneous biopsy is a critical part of the diagnosis, staging and follow up of suspected or known malignancies. Previously, a surgical biopsy was necessary to obtain sufficient tissue for making the pathologic diagnosis, but this is invasive and associated with morbidity (1). Therefore, image-guided percutaneous biopsy procedures are increasing being used due to their less invasive nature, the lower complication rate and the lower cost as compared with surgical methods.
CT-guided biopsy has been widely accepted as an effective and safe procedure to confirm the diagnosis in many clinical settings (2-5). The advantage of CT guidance is that the whole abdomen and retroperitoneum are visualized, and this allows accurate planning of a biopsy for deep-seated lesions such as retroperitoneal lymphadenopathy and it avoids damage to important organs and major vessels. In the recent years, the efficacy of CT-guided biopsy for making the pathological diagnosis of lymphoma has been reported as both the biopsy techniques and the histological diagnostic techniques have dramatically improved (6-8). Yet in cases of intraperitoneal or retroperitoneal lymphoma, there is a tendency for surgical biopsy to be performed at most institutions to obtain an adequate specimen for specification of the subtype. The purpose of this study was to retrospectively investigate the clinical procedural performance of a CT-guided needle biopsy for retroperitoneal lesions. We verified the usefulness of CT-guided biopsy for retroperitoneal lesions, which was reported in previous studies. We especially evaluated the diagnostic confirmation of the subtype of lymphoma.
Between April 1998 and June 2009, CT guided needle biopsies were performed in 74 consecutive patients (M:F = 44:30) at our hospital with retroperitoneal lesions due to the suspicion of malignant lymphoma, lymph node metastasis or primary tumor. The mean patient age was 59.7 years (range, 18-80 years). All the patients were biopsied as inpatients and they were observed for at least 12 hours after the biopsy. This retrospective review of collected data was approved by the ethics committee at our institution.
Over the same period, ultrasound (US)-guided biopsy for retroperitoneal lesions was performed for 96 patients and surgical biopsy was done for seven. Accordingly, a total of 177 biopsies were performed for determining retroperitoneal lesions.
The maximum diameter of the target lesion ranged from 1.5 to 12.5 cm in size (mean size, 5.1 cm). Of the 74 patients, the lesions were smaller than 3 cm in 27 patients, between 3 and 6 cm in 23 patients and larger than 6 cm in 24 patients.
The 74 lesions for which biopsies were performed varied widely in location. Forty nine were paraaortic, seven were retrocaval, six were within or adjacent to the psoas muscle, four were in the iliac region, three were in the presacral space and five were in other locations such as the paravertebral area. The biopsy access path ranged from 3.5 to 11.5 cm in depth (mean, 8.6 cm).
The criteria for CT-guided biopsy were 1) an undiagnosed lesion was found that clinically required a confirmative histopathologic diagnosis by biopsy, 2) the puncture path to the lesions as a safe approach was confirmed on CT and 3) consent from the patient was obtained.
The objects of CT-guided biopsy were lesions with a confirmed linear puncture path that was safe without interruption by major vessels and bowel when it was impossible to perform US-guided biopsy. Contrast enhanced CT scans were available prior to the biopsy procedure to determine the exact tumor location, the degree of vascularity and the presence of necrosis, and to avoid major vessels and bowel adjacent to the lesion.
The exclusion criterion was a bleeding tendency. The prothrombin time (PT), activated partial thromboplastin time (APTT) and platelet counts were also routinely obtained to rule out any bleeding tendency, and the relative contraindications included platelet counts < 50,000/mL, a PT > 15 sec and an APTT > 39 sec.
All 74 patients satisfied the criteria. All the biopsies were performed under conscious sedation and local anesthesia. The position of the patient was chosen on the basis of previous CT imaging and a survey CT scan was performed before the biopsy to determine the puncture site and the linear needle path as a safe approach with using a tracking scale. The biopsy procedure was performed under conventional CT guidance in nine cases and CT fluoroscopic guidance in 65 cases (Fig. 1). The tandem technique was used for the conventional CT guidance. An initial 23- or 21-gauge needle was placed and this was followed by the puncture of the biopsy needle in tandem alongside the initial needle, and as visualized by sequential CT guidance. For the CT fluoroscopy guidance, an I-I device (Hakko, Tokyo, Japan) was used, which is a needle holder, and the biopsy needle was directly punctured and advanced toward the target under intermittent CT fluoroscopy (9). Immediately after the biopsy, CT scans were routinely obtained for the assessment of acute complications such as bleeding, and the patients remained in hospital for at least 12 hours for observation to assess any delayed complications.
A biopsy specimen was obtained using an 18-gauge core biopsy needle. A Fine-Core disposable semiauto biopsy needle (Toray, Tokyo, Japan) was used in 50 cases and a Biopty-Cut disposable core biopsy needle (Bard, Covington, GA) driven by a spring-loaded Bard MAGNUM biopsy instrument was used in the remaining 24 cases. All the biopsies were performed using an interventional CT system that had a unified CT and angiography unit made by Toshiba Medical Systems (X-vigar from 1998 to 2008 or Aquilion after 2009 combined with TSX-201A).
Technical success was defined as collecting a tissue sample that was adequate for the diagnosis. The biopsy sample was determined to be either representative or nonrepresentative and the amount of biopsy material was graded either sufficient or insufficient for diagnostic use by a pathologist.
The histological results were based on the interpretation of the biopsy sample, and the sample was prepared by the standard techniques used in the pathology department of our hospital. The sample was usually analyzed with Hematoxylin and Eosin stain and other stains as required. When lymphoma was suspected, an extended panel of immunohistochemical stains was performed. The histopathological diagnosis from the CT-guided biopsies was compared with the final diagnosis made from the clinical and radiologic follow-up or the surgery, if available. The following criteria were applied for the statistical analysis of our histological results. A true positive was defined as histopathologically confirmed malignancy. A true negative was defined as there being no malignant signs in the histological samples or any remarkable change during the follow-up period. A false positive was defined as a histopathologically falsely diagnosed malignancy. A false negative was defined as cases where a lesion that was diagnosed as not being a malignancy had shown a malignant appearance such as rapid growth or invasive grown when observed on follow-up CT. Nonrepresentative or insufficient samples were also considered as false negatives. The diagnostic sensitivity, specificity and accuracy were calculated using these definitions.
The CT-guided biopsies of the retroperitoneal lesions were technically successful in all the patients. The biopsy procedure was performed with an anterior approach with the patient in the anterior oblique position for three patients and with a posterior approach with the patient in the prone position for 71 patients. Each patient received a mean of 2.0 punctures (range: 1-4 punctures) per one biopsy. The pathological diagnosis was made for 70 (95%) of 74 patients. In the remaining four cases, the sample was either nonrepresentative (n = 3) or insufficient (n = 1). These lesions were a mean of 4.1 cm in size and the biopsy access path was a mean of 10.2 cm in depth. Of the 70 cases with a pathologic diagnosis, 63 were malignant and seven were benign. Among the malignant cases, 45 were diagnosed as lymphomas, nine were diagnosed as lymph node metastases and nine were diagnosed as primary tumors. The primary tumors in the nine cases included liposarcoma in two, and rhabdomyosarcoma, malignant peripheral nerve sheath tumor, primitive neuroectodermal tumor, malignant schwannoma and local recurrence of rectal cancer in one patient each, with the remaining two cases being diagnosed as sarcoma that could not be subclassified anymore. The subtype was specified according to the WHO classification in 43 of 45 patients (96%) who were diagnosed as having lymphoma (Table 1). Among the benign seven cases, five were diagnosed as benign tumors, which were schwannoma (n = 4) and angiomyolipoma (n = 1), and the remaining two cases were reactive lymphadenopathy. No major complications defined as those requiring surgery or medical intervention were seen in any of the patients either during or after the procedure. Minor complications were seen in seven patients. Five patients developed small retroperitoneal hematomas in the psoas muscle (n = 3) and adjacent to the psoas muscle (n = 2) that were detected on CT scans just after the biopsies (Fig. 2). In these five patients, the mean number of punctures per biopsy was 2.2 times. All of the five patients were completely asymptomatic and they did not require a blood transfusion. The remaining two patients had transient pain at the puncture site with a mean 1.5 times of puncture. The CT scans just after biopsy did not reveal any marked abnormality and the pain was gradually relieved without medications.
The 70 diagnoses obtained by the biopsy were finally confirmed by surgical resection (n = 4) or clinical and imaging follow-up for more than six months (n = 66). Of the seven cases diagnosed as benign, six remained stable on follow-up CT scans that were done between nine and 92 months (median time, 40.6 months) after the CT-guided biopsy, but the remaining one was diagnosed as reactive lymphoadenopathy, and it had gradually increased in size during the follow-up and it was clinically diagnosed as lymph node metastasis of sigmoid colon cancer. The four undiagnosed lesions proved to be malignant by re-biopsy (n = 1) and follow-up imaging (n = 3).
Analysis of the value of CT-guided biopsy in this series indicated 63 true positives, zero false positives, six true negatives and five false negatives (including three nonrepresentative samples and one insufficient sample). The sensitivity was 93%, the specificity was 100% and the accuracy was 93%.
The safety of percutaneous CT-guided needle biopsy is well documented (10, 11). The mass lesions that develop in the retroperitoneal space are usually shielded by the aorta, inferior vena cava, kidney, bowel and/or other structures. CT guidance helps to identify and avoid these major structures adjacent to the target biopsy site. As the contraindications to CT-guided biopsy, the lack of patient cooperation, coagulation problems and technical impossibility due to interruption by major vessels and bowel are noted. As a rule, we decided through discussions with physicians that CT-guided biopsy was not indicated in a case that the envisaged path direction was not considered to be safe due to interruption by major vessels, bowel and vertebral bodies adjacent to the target lesions. For the case that the maximum diameter of the target lesion was less than 1 cm, we also considered that CT-guided biopsy had poor accuracy to biopsy such a small mass and there was an increased risk.
In this study, we could obtain enough specimens from the small lesions located at a deep site with the patient under local anesthesia and without major complications. Among the retroperitoneal lesions for which the US-guided approach was considered to be difficult, it was possible to perform CT-guided biopsy in 74 patients (91%), although eventually surgical biopsy was performed in seven.
A satisfactory sample for histological examination is fundamental for making the diagnosis and managing lymphomas (12). Core needle biopsies were superior to fine needle aspiration biopsies for diagnosing lymphomas because a core needle can acquire a relatively large specimen and this allows better immunohistochemical staining. It was possible to use a CT-guided biopsy to make the specific diagnosis of the lymphoma type in most cases and it played a very important role to determine the treatment modalities. Knelson et al. (13) reviewed CT-guided needle biopsy for retroperitoneal lesions, and both the diagnosis and the histological sub-typing of lymphoma could be determined in 10 of 11 cases using the 14-gauge Tru-Cut needle, but it was not possible to make the specific diagnosis in any of the lymphoma patients using the 20-gauge Chiba needle. Agid et al. (14) reported that CT-guided core needle biopsies were sufficient to establish a diagnosis in 83% of the patients with lympho-proliferative disorders and they suggested that it should be used as the first step in the diagnosis of lymphomas. Stattaus et al. (15) reported that the correct lymphoma subtype could be revealed for retroperitoneal masses in 87% of the patients by using a 16 or 18-gauge core biopsy system with the coaxial technique under CT guidance. In our study, 43 (96%) of 45 patients had a defined diagnosis and the correct histological subtype was determined via an 18-gauge core needle and the subsequent treatment was performed on the basis of the results of the biopsy. Our results might be better as compared with those of the previous reports because the development of CT fluoroscopy, which enables continuous visualization of the needle during biopsy and improved the CT-guided needle biopsy procedures (16). Cheung et al. (17) recently reported the usefulness of combined fluoroscopy- and CT-guided biopsy. De Bazelaire et al. (18) described that a coaxial introducer provided with an additional blunt-tip stylet allows safe access to difficult-to-reach lymph nodes in the chest, abdomen and pelvis under CT control. CT-guided biopsy using this blunt-tip coaxial introducer might be possible even though there are major vessels and bowel adjacent to the target lesions.
US and magnetic resonance imaging (MRI) can be used similar to other imaging equipment, in addition to CT guidance. US-guided biopsies are faster and more cost-effective than CT-guided biopsies, and US-guided biopsies have established accuracy (19, 20). However, the field of view of US-guided biopsy is smaller than that of CT, and this often makes it difficult to continuously localize retroperitoneal lesions and to sufficiently control the needle track (21). MRI, similar to US, has no influence of irradiation. MRI has recently developed into a potent alternative modality for guiding such procedures and there are already numerous reports of different procedures, including abdominal biopsies guided by MRI (22-25). Zangos et al. (24) described that MRI guidance to perform retroperitoneal biopsy should be justified based on a better practicability of the biopsy procedure with its superior soft tissue contrast and multiplanar imaging capabilities, and infants would especially benefit from MRI-guided biopsy due to the lack of a radiation burden (23). But there are technical difficulties involved in punctures that require the use of open-configuration MRI techniques (24), and the cost of MRI-guided biopsy is more expensive compared with that of CT-guided biopsy due to the higher material costs for the MRI-compatible biopsy needles (25). MRI guidance lacks real-time imaging and this might lead to a prolonged and cumbersome procedure in an incremental fashion (24). We have no open-configuration MRI system and we won't talk about it any more. We believe the CT-guided approach is better for retroperitoneal lesions because of its wide field of view and clear visualization, although the CT-guided approach involves radiation exposure.
This study has some limitations such as a case-selection bias. We did not know the real number of retroperitoneal lesions that required a pathologic diagnosis at our hospital. We did not perform biopsy for every retroperitoneal lesion that required a pathologic diagnosis. We eventually decided in advance whether biopsy could be performed safely, on the basis of CT findings, after physicians thought it might be possible to perform a CT-guided biopsy for the lesion. It remains unclear whether a CT-guided biopsy for every retroperitoneal lesion is achievable.
In conclusion, our results indicate that the technique of CT-guided needle biopsy provides accurate and safe access to retroperitoneal lesions because of its high success and low complication rate. This technique is highly practical and useful and particularly for patients with suspected lymphoma.
Figures and Tables
Fig. 1
53-year-old man with paraaortic lesion.
A. Diagnostic axial contrast enhanced CT scan that was obtained prior to biopsy procedure with patient in supine position shows 2-cm paraaortic mass lesion (arrow). B. Initial axial CT scan obtained with patient in prone position shows tracking scale crossing retrocaval mass lesion.
Distance from skin to leading edge of lesion was 10 cm. C. CT fluoroscopic image obtained during biopsy procedure shows safe insertion of biopsy needle (arrow) into lesion. Pathologic diagnosis was nodular sclerosis of Hodgkin's disease. D. CT scan immediately after two punctures reveals no complications.
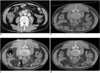
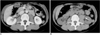
Fig. 2
56-year-old man with paraaortic lesion.
A. Diagnostic axial contrast enhanced CT scan obtained prior to biopsy procedure with patient in supine position shows 3-cm paraaortic mass lesion (arrow). B. CT scan immediately after two punctures by posterior approach for biopsy reveals retroperitoneal hematoma (arrow).
References
1. Hopper KD. Percutaneous, radiographically guided biopsy: a history. Radiology. 1995. 196:329–333.
2. Gazelle GS, Haaga JR. Guided percutaneous biopsy of intraabdominal lesions. AJR Am J Roentgenol. 1989. 153:929–935.
3. Ben-Yehuda D, Polliack A, Okon E, Sherman Y, Fields S, Lebenshart P, et al. Image-guided core-needle biopsy in malignant lymphoma: experience with 100 patients that suggests the technique is reliable. J Clin Oncol. 1996. 14:2431–2434.
4. Guimaraes AC, Chapchap P, de Camargo B, Chojniak R. Computed tomography-guided needle biopsies in pediatric oncology. J Pediatr Surg. 2003. 38:1066–1068.
5. Husband JE, Golding SJ. The role of computed tomography-guided needle biopsy in an oncology service. Clin Radiol. 1983. 34:255–260.
6. Sklair-Levy M, Polliack A, Shaham D, Applbaum YH, Gillis S, Ben-Yehuda D, et al. CT-guided core-needle biopsy in the diagnosis of mediastinal lymphoma. Eur Radiol. 2000. 10:714–718.
7. Demharter J, Muller P, Wagner T, Schlimok G, Haude K, Bohndorf K. Percutaneous core-needle biopsy of enlarged lymph nodes in the diagnosis and subclassification of malignant lymphomas. Eur Radiol. 2001. 11:276–283.
8. Pappa VI, Hussain HK, Reznek RH, Whelan J, Norton AJ, Wilson AM, et al. Role of image-guided core-needle biopsy in the management of patients with lymphoma. J Clin Oncol. 1996. 14:2427–2430.
9. Irie T, Kajitani M, Matsueda K, Arai Y, Inaba Y, Kujiraoka Y, et al. Biopsy of lung nodules with use of I-I device under intermittent CT fluoroscopic guidance: preliminary clinical study. J Vasc Interv Radiol. 2001. 12:215–219.
10. Welch TJ, Sheedy PF 2nd, Johnson CD, Johnson CM, Stephens DH. CT-guided biopsy: prospective analysis of 1,000 procedures. Radiology. 1989. 171:493–496.
11. Chojniak R, Isberner RK, Viana LM, Yu LS, Aita AA, Soares FA. Computed tomography guided needle biopsy: experience from 1,300 procedures. Sao Paulo Med J. 2006. 124:10–14.
12. Libicher M, Noldge G, Radeleff B, Gholipur F, Richter GM. Value of CT-guided biopsy in malignant lymphoma. Radiologe. 2002. 42:1009–1012.
13. Knelson M, Haaga J, Lazarus H, Ghosh C, Abdul-Karim F, Sorenson K. Computed tomography-guided retroperitoneal biopsies. J Clin Oncol. 1989. 7:1169–1173.
14. Agid R, Sklair-Levy M, Bloom AI, Lieberman S, Polliack A, Ben-Yehuda D, et al. CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol. 2003. 58:143–147.
15. Stattaus J, Kalkmann J, Kuehl H, Metz KA, Nowrousian MR, Forsting M, et al. Diagnostic yield of computed tomography-guided coaxial core biopsy of undetermined masses in the free retroperitoneal space: single-center experience. Cardiovasc Intervent Radiol. 2008. 31:919–925.
16. Carlson SK, Felmlee JP, Bender CE, Ehman RL, Classic KL, Hoskin TL, et al. CT fluoroscopy-guided biopsy of the lung or upper abdomen with a breath-hold monitoring and feedback system: a prospective randomized controlled clinical trial. Radiology. 2005. 237:701–708.
17. Cheung JY, Kim Y, Shim SS, Lim SM. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol. 2011. 12:89–96.
18. de Bazelaire C, Farges C, Mathieu O, Zagdanski AM, Bourrier P, Frija J, et al. Blunt-tip coaxial introducer: a revisited tool for difficult CT-guided biopsy in the chest and abdomen. AJR Am J Roentgenol. 2009. 193:W144–W148.
19. Memel DS, Dodd GD 3rd, Esola CC. Efficacy of sonography as a guidance technique for biopsy of abdominal, pelvic, and retroperitoneal lymph nodes. AJR Am J Roentgenol. 1996. 167:957–962.
20. al-Mofleh IA. Ultrasound-guided fine needle aspiration of retroperitoneal, abdominal and pelvic lymph nodes. Diagnostic reliability. Acta Cytol. 1992. 36:413–441.
21. Adam G, Bucker A, Nolte-Ernsting C, Tacke J, Gunther RW. Interventional MR imaging: percutaneous abdominal and skeletal biopsies and drainages of the abdomen. Eur Radiol. 1999. 9:1471–1478.
22. Lee MH, Lufkin RB, Borges A, Lu DS, Sinha S, Farahani K, et al. MR-guided procedures using contemporaneous imaging frameless stereotaxis in an open-configuration system. J Comput Assist Tomogr. 1998. 22:998–1005.
23. Kariniemi J, Blanco Sequeiros R, Ojala R, Tervonen O. MRI-guided abdominal biopsy in a 0.23-T open-configuration MRI system. Eur Radiol. 2005. 15:1256–1126.
24. Zangos S, Eichler K, Wetter A, Lehnert T, Hammerstingl R, Diebold T, et al. MR-guided biopsies of lesions in the retroperitoneal space: technique and results. Eur Radiol. 2006. 16:307–312.
25. Alanen J, Keski-Nisula L, Blanco-Sequeiros R, Tervonen O. Cost comparison analysis of low-field (0.23 T) MRI- and CT-guided bone biopsies. Eur Radiol. 2004. 14:123–112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download