Abstract
Objective
We wanted to differentiate between transient ischemic attack (TIA) and minor stroke using fractional anisotropy and three-dimensional (3D) fiber tractography.
Materials and Methods
The clinical data, conventional magnetic resonance imaging (MRI), diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI) were obtained for 45 TIA patients and 33 minor stroke patients. The fractional anisotrophy ratio (rFA) between the lesion and the mirrored corresponding contralateral normal tissue was calculated and analyzed. The spatial relationship between the lesion and the corticospinal tract (CST) was analyzed and the lesion sizes in the minor stroke patients and TIA patients were compared.
Results
Twenty-two of the 45 TIA patients (49%) revealed focal abnormalities following DWI. The rFA was significantly lower (p < 0.05) in the stroke patients (0.71 ± 0.29) compared to that of the TIA patients (1.05 ± 0.37). The CST was involved in almost all stroke lesions, but it was not involved in 68% of the TIA lesions. The TIA patients had significantly lower CST injury scores (3.25 ± 1.75) than did the stroke patients (8.80 ± 2.39) (p = 0.004).
Much interest has recently been given to the potential usage of diffusion tensor imaging (DTI) for the in vivo quantification of microstructural damage to the cerebral white matter following stroke. DTI provides information about the predominant direction and degree of water diffusion in tissues (1, 2). In the white matter, water diffuses quickly lengthwise along the fibers and slowly perpendicular to fibers, resulting in anisotropic diffusion (3). The degree of anisotropy depends on the level of organization and the integrity of the white matter tract and on the degree of freedom of water diffusion movement caused by the oriented axonal membranes and myelin sheaths (4). Reduced anisotropy along the corticospinal tract (CST) remote from a cerebral infarct has been interpreted as Wallerian degeneration (WD) (5, 6). DTI can quantify the fractional anisotropy (FA) values to evaluate the pathology change of the cerebral white matter, and the FA values are valuable for detecting WD and evaluating the prognosis.
Diffusion-weighted imaging (DWI) revealed that focal abnormalities existed not only in stroke patients, but also in up to 67% of the patients with a clinical transient ischemic attack (TIA) (7-13). Winbeck et al. (14) indicated that within six hours after symptom onset, TIA and stroke could be differentiated by analyzing the DWI signal intensity of the lesions. However, to the best of our knowledge, a controlled study concerning differentiating TIA from stroke by using FA values is yet to be conducted. Therefore, we have investigated the possibility of differentiating TIA from minor stroke by analyzing the FA values and the DTI within 24 hours after symptom onset.
The study groups were 78 patients who were admitted to Huashan hospital from June 2006 to July 2007 and who were clinically diagnosed as suffering a TIA or minor stroke by neurologists and according to follow-up MRI. TIA was defined as an acute transient focal neurological deficit caused by vascular disease and the deficit was completely reversed within 24 hours. Minor stroke was defined as a National Institutes of Health Stroke Scale (NIHSS) score ≤ 5. The relevant criteria (for this sub-study) were as follows. 1) The exact onset of symptoms was available for all cases. 2) The TIA patients whose clinical symptoms were most likely referable to one of the cerebral hemispheres were included (the patients with clinical symptoms referable to the posterior fossa were excluded). Minor stroke patients with brain stem or cerebellar infarction were excluded. 3) The DWI and DTI sequences were performed within 24 hours after symptom onset. 4) The clinical and demographic variables, as well as information regarding age, gender, the symptom duration and the cardiovascular risk factors (e.g., coronary artery disease, hypertension, diabetes, hypercholesterolemia and atrial fibrillation) were gathered from the chart of every patient. 5) None of the patients had treatment with thrombolysis (intravenous or intraarterial), percutaneous angioplasty, mechanical disruption of the occluding thrombus or induced hypertension before MRI. 6) Acute DWI lesions were present on one cerebral hemisphere while the other hemisphere was normal. 7) The patients had no other cerebral diseases (e.g., hemorrhage, tumor, etc.) or a history of cerebral operations. 8) There were no constructed defects on the DTI images caused by head motion.
The MRI scans were acquired using the 3.0T GE Signa Horizon scanner (Signa Horizon, GE Medical Systems, Milwaukee, WI) with equipped with an enhanced gradient system. For this study the following sequences were evaluated: conventional T1, axial fast-spin-echo T2-weighted and axial fluid-attenuated inversion-recovery (FLAIR). DWI was performed using a single shot-spin echo-planar imaging sequence with a repetition time (TR)/echo time (TE): 4800/min ms, a slice thickness of 6 mm, a 128 × 128 matrix and a 240 mm field of view, which involved a b value of 1000 s/mm2 in each of the three orthogonal directions; DTI was performed using a single shot-spin echoecho planar imaging sequence with a TR/TE: 10,000/112 min ms, a slice thickness of 5 mm, a 128 × 128 matrix and a 220 mm field of view, which involved b values of 0 and 1000 s/mm2 in each of the 21 orthogonal directions. The study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University, and each participant signed the informed consent form.
The original DTI images were transferred to a SUN ADW 2.0 work station to rebuild the FA images and the white matter tracts using Functool 2 software. The ratio of the lesion to the corresponding normal contralateral tissue average signal intensity (rAI) on b = 1000 images (rAIb = 1000) was calculated. The quantitative FA values of the corresponding hypointense or hyperintense lesions and the values of the corresponding normal contralateral tissue were analyzed. To control the differences in absolute FA values at any given scan and thereby permit more meaningful comparisons between groups, we used the FA ratio (rFA) of the lesion to the mirrored contralateral normal tissue. If multiple lesions or noncontiguous abnormalities were noted, then the ratio was calculated by summing the individual average intensities of all the abnormal lesions and dividing the results by the average intensity of the corresponding mirrored contralateral normal brain areas.
Three seeds were used to reconstruct the CST. 2D FA maps and colorized directional encoded color (DEC) maps were acquired simultaneously through this process. The first region of interest (ROI) was placed on the cerebral peduncle, the second on the posterior limb of the internal capsule and the third on the anterior central gyrus and part of the paracentral lobule. The conditions for fiber tracking were 0.18 < FA value < 1.00, 0.00 < apparent diffusion coefficient (ADC) value < 0.01 and 0.00 < S0 < 1000.0. The time for constructing both sides of the CST was 10-15 minutes. The spatial relationship between the DWI lesion and the CST was analyzed in multiple angles by combining the 3D CST with the FA and DEC maps to reveal the degree of CST injury. Scores that were arrived at according to the following criteria were used to evaluate the degree of CST injury: 1) in cases where the DWI lesion was completely located outside the CST, the CST injury was scored 0 (i.e., integrated CST), 2) when the DWI lesion was partially located inside the CST (i.e., the CST was partially involved), the CST injury was scored 1 denoting CST injury ≤ 1/3, the CST injury was scored 2 indicating 1/3 < CST injury ≤ 2/3 and it was scored 3 indicating 2/3 < CST injury < 1 and 3) a CST injury score of 4 indicated that the DWI lesion was entirely located inside the CST (i.e., the CST was completely involved). When the scores for each and every slice were known, the exact degree of CST injury could be identified. Note: the lesion volume was measured by adding the DWI lesion area according to the following formula: Σ (slice thickness + interval thickness).
Al the values are given as a mean and the 95% confidence interval (CI). Between-group comparisons were performed with X2 and Student's t tests for the normally distributed data. P values < 0.05 were considered significant. Receiver operating characteristic (ROC) curves were constructed and the optimum cutoff values with the best combination of sensitivity and specificity were calculated. The overall accuracy of the diagnosis was expressed by the area under the curve, and this ranged from 0.5 to 1. A value of one implied perfect sensitivity and specificity, whereas a value of 0.5 implied that the model's accuracy was no better than chance (15). Generally, a value > 0.7 could be interpreted as reasonable and a value > 0.8 indicated good accuracy (16). The statistical analysis was performed using SPSS 13.0.
Forty-five TIA patients (M:F = 35:12; average age, 59.6) and 33 stroke patients (M:F = 16:17; average age, 65.2) were enrolled. Twenty-two out of the 45 TIA patients (49%) revealed focal abnormalities following DWI. The signal intensity was significantly higher (p = 0.008) in the minor stroke patients (n = 33, rAIb = 1000 = 1.96) when compared with that of the TIA patients with DWI lesions (n = 22, rAIb = 1000 = 1.46), while the rFA was significantly lower (p = 0.002) in the minor stroke patients (n = 33, rFA = 0.71) when compared with that of the TIA patients with DWI lesions (n = 22, rFA = 1.05) (Fig. 1) (Table 1). Among the 22 TIA patients with DWI lesions, 12 (55%) showed increased FA values and the mean rFA value was 1.21 ± 0.16.
Diffusion tensor imaging was performed for three groups of patients after different lengths of time of symptom onset: first on 16 (36%) TIA and 17 (52%) stroke patients within six hours after symptom onset, then on nine (20%) TIA and nine (27%) stroke patients between six and 12 hours after symptom onset and finally on 20 (44%) TIA and seven (21%) stroke patients between 12 and 24 hours after symptom onset. Eight TIA patients in the first group (50%), five in the second group (56%) and nine in the third group (45%) showed DWI abnormalities. At all the time intervals, the rAIb = 1000 and rFA were significantly different for the stroke patients when compared with those values of the TIA patients (Fig. 2). Six TIA patients in the first group (50%), four in the second group (33%) and two in the third group (17%) showed increased FA values.
Receiver operating characteristic curve analysis revealed a good value for the area under the curve (0.86 for the rFA) (Fig. 3). For an rFA > 0.9, the sensitivity for TIAs was 75%, with a specificity of 73% and a positive predictive value of 72%. For the patient group in which the DTI was performed within six hours after symptom onset (8 TIA patients and 17 stroke patients), the area under the curve was 0.77 (95% CI: 0.78, 1.25; p = 0.03) for the rFA. The sensitivity and specificity of the rFA were 70% and 65%, respectively.
The spatial relationship between the ischemic lesion and the CST can be categorized into three types: integrated CST, partially involved CST and completely involved CST. For the 22 TIA patients with DWI lesions, 68% (15 of 22) had an integrated CST, 32% (7 of 22) had a partially involved CST and none had a completely involved CST, whereas for 33 stroke patients, none had an integrated CST, 46% (15 of 33) had a partially involved CST and 55% (18 of 33) had a completely involved CST. The TIA patients (3.25 ± 1.75) had significantly lower CST injury scores than those of the stroke patients (8.80 ± 2.39) (p = 0.004). In the TIA patients, 91% (20 of 22) of the lesions were detected in the centrum ovale, corona radiate, thalamus and cortex, while 88% (29 of 33) of the lesions were detected in the internal capsule of the stroke patients. There was no difference in lesion volume between the TIA patients (5.23 ± 4.28 cm3) and stroke patients (5.63 ± 5.46 cm3) (p = 0.455) (Figs. 4, 5, 6) (Table 1).
This study has demonstrated that patients with minor stroke or TIA can be differentiated by analyzing the FA value following DTI. The DWI signal intensity was significantly higher in the minor stroke patients when compared with that of the TIA patients with DWI lesions, which agrees with the data from Winbeck et al. (14). To the best of our knowledge, this is the first study reporting differences in intensities on the FA maps of stroke patients when compared with that of TIA patients with DWI abnormalities. In addition, we were able to differentiate between TIA and stroke using the DTI data and the rFA cutoff values. In our opinion, the results of our study will be valuable for clinical practice given the timeliness of differentiating TIA from a stroke. We have shown that TIA can be distinguished from stroke by analyzing the value of the average intensity on the FA images within six hours of symptom onset. Another critical finding of our study is the accuracy by which TIA can be discriminated from stroke by measuring the rFA. Furthermore, the area under the ROC curve was > 0.8 and this revealed its high predictive capability. In addition, further studies are required to verify the discrimination power discovered in this study and empower its further implications.
Our study found that the FA values in some TIA patients were increased and became higher than the FA values of the undamaged side within 24 hours of symptom onset. The shorter the length of symptom onset to DTI, the higher the incidence of an increased FA value. Generally, the increase of anisotropy indicates water content flowing into cells from the outside without cell membrane destruction, or said a different way, the increase of diffusion anisotropy in turn indicates the integrity and continuity of the structure (17, 18). A previous study has shown that after the recovery of diffusion hyperintensity, the increased diffusion anisotropy disappeared (19). Thus, the increase or invariability of diffusion anisotropy always indicated light or early ischemic injury, which was most probably reversible and there was a high chance these patients had a good prognosis after therapy, whereas a decrease of anisotropy indicated irreversible ischemia and destruction of cell integrity. The relative increase of ecto-cellular free water caused by vascular edema and cell membrane destruction would mainly cause the decrease of diffusion anisotropy. Thus, the early decrease of diffusion anisotropy indicated irreversible ischemia and these patients had a poor prognosis after therapy even within six hours after symptom onset. In our study, the rFA of the ischemic lesions was significantly higher in the TIA patients compared with that of the stroke patients, which to some extent reflected the pathophysiological change of ischemic lesion in TIA patients, and this rFA value was different from that of stroke patients.
We also found that the degree of CST involvement in TIA patients with DWI lesions was significantly different from that of stroke patients. The lesions in stroke patients were always located in the center of the CST, while the lesions in TIA patients were always located on the side of the CST, though we found no difference in lesion volume between the TIA patients and the stroke patients. Two different infarcts of the same size in different locations could have very different functional expressions. Thus, rather than using the total stroke lesion volume, it seems much more reliable to use DTI to evaluate the extent of damage within the CST to determine the axonal injury. The tiny lesions located in the center of the CST were mostly stroke lesions, whereas the lesions near the CST, in spite of their volume, were mostly associated with TIAs. The lesions in TIA patients are almost always located in the centrum oval, corona radiate, thalamus and cortex, while the lesions in stroke patients are mostly located in the internal capsule. After performing research on 14 micro-infarction lesions involving the supratentorial white matter by employing fiber tractography, which has a sensitivity of 100% and a specificity of 77% for making the diagnosis of sensation and motion symptoms, Yamada et al. (20) found that infarction symptoms were highly correlated to the lesion location. However, they did not quantify the degree of involvement of the sensation and motion pathway.
In summary, TIA and minor stroke may be differentiated by analyzing the rFA value of the lesions and the degree of CST involvement, and this may allow a more accurate prediction of the prognosis. A fiber tracking technique that can directly display the CST will provide a new direction for WD research after cerebral ischemia. The quality of our fiber tractography was satisfactory and the time taken for data collection and processing was acceptable, although many technical problems still need to be resolved. Several potential limitations of our study merit comment. First, this study was performed from 2006 to 2007 and at that time the current definition of TIA, which defines TIA as a transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischemia, and without acute infarction (21), had not been published. Our definition of TIA at that time is reasonably sound, and we found that the DWI lesions in some patients were reversible in our study (22). In the future, more studies should be carried out to compare the TIA patients with reversible DWI lesions to minor stroke patients with bearing in mind this modification of the TIA definition. Second, it would be interesting to determine other anisotropic parameters, such as the mean diffusivity or the diffusion tensor eigenvalues, and to analyze the difference between minor stroke and TIA patients. On the other hand, all the ischemic lesions were small because the small infarction lesions had a clear spatial relation with the CST. Finally, the small number of patients could be another limitation. A larger sample size would increase the accuracy of our findings.
Figures and Tables
Fig. 1
Diffusion weighted imaging (A) and fractional anisotrophy (B) measurement in patient with transient ischemic attack (64-year-old man with transient right extremity weakness. Yet neurologic deficits were completely resolved in 6 h). Diffusion weighted imaging (C) and fractional anisotrophy (D) measurement in patient with stroke (50-year-old man with dysarthria and right extremity weakness). In transient ischemic attack patient, rAIb = 1000 was 1.58 and rFA was 0.89. In stroke patient, rAIb = 1000 was 2.25 and rFA was 0.57. rAIb = 1000 was significantly lower in transient ischemic attack patients compared with that of stroke patients, while rFA was significantly higher in transient ischemic attack patients compared with that of stroke patients. rAI = average signal intensity, rFA = fractional anisotrophy ratio
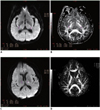
Fig. 2
Percentile rank map of rAIb = 1000 (A) and rFA (B) between transient ischemic attack and stroke patients at different time intervals. At all time intervals, rAIb = 1000 and rFA were significantly different in stroke patients as compared with those values in transient ischemic attack patients. rAI = average signal intensity, rFA = fractional anisotrophy ratio, TIA = transient ischemic attack

Fig. 3
Receiver operating characteristic curve analysis for rFA of transient ischemic attack and stroke patients.
Area under curve shows good accuracy (0.86) for discriminating transient ischemic attack from stroke using rAI measurement on fractional anisotrophy maps. rAI = average signal intensity, rFA = fractional anisotrophy ratio, ROC curve = receiver operating characteristic curve, TIA = transient ischemic attack

Fig. 4
Diffusion weighted imaging and diffusion tensor imaging scans from transient ischemic attack patient (53-year-old man with transient right hemisensory loss, weakness and dysarthria. Yet neurologic deficits completely resolved in 2-4 hours.)
A. Axial diffusion weighted imaging showed small hyperintense lesion near left cornu posterius ventriculi lateralis. B, C. Lesion volume was 2.87 cm3. Fiber tracts reconstructed in 3-dimensional space were superimposed on fractional anisotrophy map (B) and directional encoded color map (C). D, E. Fiber tract results showed corticospinal tract (orange) of affected cerebral hemisphere appeared near lesion, but it was not running through it.
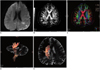
Fig. 5
Diffusion weighted imaging and diffusion tensor imaging scans from stroke patient (62-year-old man with right extremity weakness).
A, B. Axial diffusion weighted imaging (A) and apparent diffusion coefficient (B) showed small hyperintense lesion on left thalamus and internal capsule. Lesion volume was 3.12 cm3. C, D. Fiber tracts reconstructed in 3-dimensional space were superimposed on fractional anisotrophy map (C) and directional encoded color map (D). E. Fiber tract results showed left corticospinal tract (orange) was only partially involved.
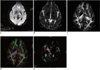
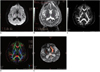
Fig. 6
Diffusion weighted imaging and diffusion tensor imaging scans from stroke patient (66-year-old man with left extremity weakness).
A, B. Axial diffusion weighted imaging (A) and apparent diffusion coefficient (B) showed small hyperintense lesion on right thalamus and internal capsule. Lesion volume was 2.86 cm3. C, D. Fiber tracts reconstructed in 3-dimensional space were superimposed on fractional anisotrophy map (C) and directional encoded color map (D). E. Fiber tract results revealed lesion was fully involved in right corticospinal tract (orange).
Acknowledgment
We acknowledge and heartily thank Dr. Wang Fei, Dr. Zhang Fang, Dr. Sun Huaping and Dr. Jiang Shenghong of Huashan Hospital Affiliated to Fudan University.
References
1. Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008. 29:632–641.
2. Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am J Neuroradiol. 2008. 29:843–852.
3. Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001. 13:1174–1185.
4. Virta A, Barnett A, Pierpaoli C. Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 1999. 17:1121–1133.
5. Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004. 22:1767–1774.
6. Ahlhelm F, Reith W. Modern diagnosis in acute cerebral infarct. Diffusion weighted imaging and ADC (apparent diffusion coefficient) calculations. Nervenarzt. 2002. 73:736–774.
7. Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke. 2003. 34:932–937.
8. Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999. 30:1174–1180.
9. Ay H, Oliveira-Filho J, Buonanno FS, Schaefer PW, Furie KL, Chang YC, et al. 'Footprints' of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc Dis. 2002. 14:177–186.
10. Marx JJ, Mika-Gruettner A, Thoemke F, Fitzek S, Fitzek C, Vucurevic G, et al. Diffusion weighted magnetic resonance imaging in the diagnosis of reversible ischaemic deficits of the brainstem. J Neurol Neurosurg Psychiatry. 2002. 72:572–575.
11. Kastrup A, Schulz JB, Mader I, Dichgans J, Kuker W. Diffusion-weighted MRI in patients with symptomatic internal carotid artery disease. J Neurol. 2002. 249:1168–1174.
12. Rovira A, Rovira-Gols A, Pedraza S, Grive E, Molina C, Alvarez-Sabin J. Diffusion-weighted MR imaging in the acute phase of transient ischemic attacks. AJNR Am J Neuroradiol. 2002. 23:77–83.
13. Lecouvet FE, Duprez TP, Raymackers JM, Peeters A, Cosnard G. Resolution of early diffusion-weighted and FLAIR MRI abnormalities in a patient with TIA. Neurology. 1999. 52:1085–1087.
14. Winbeck K, Bruckmaier K, Etgen T, von Einsiedel HG, Rottinger M, Sander D. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke. 2004. 35:1095–1099.
15. Faraggi D, Reiser B. Estimation of the area under the ROC curve. Stat Med. 2002. 21:3093–3106.
16. Nagai Y, Kitagawa K, Yamagami H, Kondo K, Hougaku H, Hori M, et al. Carotid artery intima-media thickness and plaque score for the risk assessment of stroke subtypes. Ultrasound Med Biol. 2002. 28:1239–1243.
17. Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999. 212:785–792.
18. Zelaya F, Flood N, Chalk JB, Wang D, Doddrell DM, Strugnell W, et al. An evaluation of the time dependence of the anisotropy of the water diffusion tensor in acute human ischemia. Magn Reson Imaging. 1999. 17:331–348.
19. Duong TQ, Ackerman JJ, Ying HS, Neil JJ. Evaluation of extra- and intracellular apparent diffusion in normal and globally ischemic rat brain via 19F NMR. Magn Reson Med. 1998. 40:1–13.
20. Yamada K, Mori S, Nakamura H, Ito H, Kizu O, Shiga K, et al. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003. 34:E159–E162.
21. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009. 40:2276–2293.
22. Tong T, Yao Z, Feng X. Combined diffusion- and perfusion-weighted imaging: a new way for the assessment of hemispheric transient ischemic attack patients. Int J Dev Neurosci. 2011. 29:63–69.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download