Abstract
Liver fluke disease is a chronic parasitic inflammatory disease of the bile ducts. Infection occurs through ingestion of fluke-infested, fresh-water raw fish. The most well-known species that cause human infection are Clonorchis sinensis, Opisthorchis viverrini and Opisthorchis felineus. Adult flukes settle in the small intrahepatic bile ducts and then they live there for 20-30 years. The long-lived flukes cause long-lasting chronic inflammation of the bile ducts and this produces epithelial hyperplasia, periductal fibrosis and bile duct dilatation. The vast majority of patients are asymptomatic, but the patients with heavy infection suffer from lassitude and nonspecific abdominal complaints. The complications are stone formation, recurrent pyogenic cholangitis and cholangiocarcinoma. Approximately 35 million people are infected with liver flukes throughout the world and the exceptionally high incidence of cholangiocarcinoma in some endemic areas is closely related with a high prevalence of liver fluke infection. Considering the impact of this food-borne malady on public health and the severe possible clinical consequences, liver fluke infection should not be forgotten or neglected.
The general term 'fluke' refers to flat fish, for example, rays in the sea or leeches in fresh-water. Like leeches, liver flukes are flat helminthes or platyhelminth, of the class trematoda, and they reside in the human bile ducts (Fig. 1). Infection occurs through ingestion of fluke-infested, fresh-water raw fish. It is one of the most important food-borne diseases. Throughout the world, particularly in South-East Asia, China, Manchuria, the former USSR and northern Siberia, more than 35 million people including 15 million Chinese (1) are infected. The epidemiology of the infections is determined by food and ecological factors and it is strongly influenced by poverty and pollution in some areas and by the traditions of food intake in other areas.
The disease is generally dormant and the infected people are asymptomatic, except for the patients with very heavy infections. However, the long-lived flukes cause chronic inflammation of the bile ducts, leading to suppurative cholangitis, formation of bile duct stone and the development of cholangiocarcinoma.
In this article, we review the parasitology, epidemiology and the clinical findings and complications of liver fluke infection, and particularly the pathogenesis of cholangiocarcinoma in association with liver flukes.
Clonorchiasis and opisthorchiasis are trematodiases that are caused by chronic infection of the human liver flukes in the biliary tree. The three species of flukes, Clonorchis sinensis, Opisthorchis viverrini and Opisthorchis felineus are closely related trematodes that have similar life cycles and they cause the same pathophysiology to the biliary tract. The flukes differ in their geographic distribution and morphology.
The adult fluke of Clonorchis sinensis is a willow-leaflike, flat, flabby worm that lives in the biliary tree (Fig. 2). The size of the parasite ranges from 8.0 to 15.0 mm long by 1.5 to 4.0 mm wide and 1.0 mm thick (2). Humans are infected when ingesting uncooked fresh water fish infested with metacercariae. The larvae excyst in the stomach, migrate to the ampulla of Vater, ascend into the bile ducts and live there for 20-30 years. Usually they reside in the medium sized or small intrahepatic bile ducts, but they may also live in the extrahepatic duct, gallbladder and the peripheral pancreatic branch ducts. Intraductal flukes cause mechanical obstruction, inflammation, epithelial adenomatous hyperplasia and periductal fibrosis (2-5) (Fig. 2). One adult fluke lays 2000-4000 eggs each day and the eggs are excreted through the bile ducts and feces (2). The cycle recirculates via eating of raw fresh-water fish.
The adult fluke of Opisthorchis is very similar to C. sinensis in morphology. As compared to C. sinensis, O. viverrini and O. felineus are slightly smaller at approximately half the size and their testes are less branched (6). As the endemic areas of Opisthorchis are different from Clonorchis, the first and second intermediate hosts are slightly different. However, the pathophysiology, clinical manifestations and imaging findings of O. viverrini and O. felineus infection are the same as those of C. sinensis (7-9).
The clinical symptoms depend on the degree of infection and the presence of complications. The vast majority of patients are asymptomatic. In patients with severe infection, the clinical signs and symptoms include lassitude (Fig. 3), hepatomegaly and nonspecific abdominal complaints such as anorexia, nausea, vomiting, abdominal discomfort and indigestion (2, 8-10). Jaundice is due to the mechanical obstruction caused by a multitude of flukes in the bile ducts in patients with a heavy infection, or it is due to bile duct obstruction caused by stone, cholangitis or cholangiocarcinoma as a late complication of chronic infection. The public health impact and economic impact are considerable in terms of the morbidity, the loss of productivity and the absenteeism of heavily infected people (1).
The geographic distribution of liver flukes is largely in Asia and Eastern Europe. Endemic areas of Clonorchis sinensis include east Russia and Manchuria, South Korea, mainland China (except the northwest), Taiwan and northern Vietnam (1-3), whereas the endemic areas of Opisthorchis viverrini include Laos and northeast Thailand (1-4). The endemic areas of Opisthorchis felineus include Eastern Europe and the former U.S.S.R. People living along rivers are prone to infection by flukes because they have a habit of eating uncooked fresh-water fish. In southern China, eastern Manchuria and northern Thailand, millions of people are patients with liver fluke disease, and in some endemic areas, 30-75% of the inhabitants are infected (1-4). In the North American countries where many Asian immigrants are living, there are some people infected with C. sinensis and O. viverrini. It is estimated that about 35 million people are infected throughout the world (1-3).
In China, there are two principal endemic areas, namely southern China and Manchuria. Depending upon the endemic areas, the prevalence rate is variable at between 10% and 85%, and average prevalence rate in southern China is 18% (11). There are about 8.5 million people infected in the Guangdong and Guangxi areas and another several million people in Manchuria (11, 12).
In northern Thailand and low land Laos, there is a long tradition of eating raw fresh-water fish and salt-fermented fish. Cyprinoid fish, which are fresh water fish, are an important source of protein in these areas (1). It has been reported that average prevalence rate of opisthorchiasis in northern Thailand is 22% and 5 million of the 19 million people in this region are infected (13). The infection rate increases among the people who are living in endemic areas, ranging from 60 to 80% depending upon the communities (14). In a community in Khon Kaen, the overall prevalence of O. viverrini infection is 94% and it reaches 100% among the people above the age of 10 years (15). The tradition of eating raw or undercooked fish products is the reason for this high prevalence (14).
The prevalence rate of C. sinensis infection in South Korea is 3%; it is 2% in urban areas and 5% in rural areas (16). In some regions along some rivers, the prevalence rate is about 25-30%. In a hospital based controlled study, based on radiological study and a history of eating raw freshwater fish, about 30% of hospitalized adults (31-79 years old) had evidence of clonorchiasis (17). This high prevalence rate is because the patients included in the study were hospitalized, aged patients including long-lasting infection as well as healed infection.
Bile stagnation due to mechanical obstruction caused by epithelial hyperplasia and the profuse mucin caused by goblet cell hyperplasia make the bile ideal for stone formation. The presence of worms and ova play a role as the nidus for the formation of stones (2). Bacterial deconjugation of bilirubin-glucuronide with formation of insoluble bilirubin during bouts of cholangitis may precipitate formation of bilirubin stone (18). Not infrequently, C. sinensis eggs are found in gallstones (19) (Fig. 4). In a hospital-based study based on sampling of bile, C. sinensis egg positive rate was 26% in patients with gallbladder and bile duct stones (20). In another hospital based case-control study in Korea, radiologic evidence of C. sinensis infection was significantly associated with development of intrahepatic stones (21) (Fig. 5).
Recurrent pyogenic cholangitis is the most common complication of liver fluke infection. Acute suppurative cholangitis may be caused by blockage of the extrahepatic bile ducts by masses of dead worms, ova and mucin, and this in turn results in ascending cholangitis (2, 22). When the bile flow is hampered by the presence of flukes per se, their ova, the excreta of the flukes and mucin, then ascending bacterial infection usually follows. The bacteria is Escherichia coli in the majority of patients (22). With repeated suppurative infection, the bile duct is filled with pus and the liver flukes die because of an unfavorable living environment, and the dead flukes are expelled through the ampulla of Vater (2). The egg positive rate is significantly decreased in patients with suppurative cholangitis, compared to that of the uninfected patients in the same cohort (22).
Hepatocellular carcinoma and cholangiocarcinoma are the two most common primary malignant tumors arising in the hepatobiliary system. While hepatocellular carcinoma is well recognized globally, cholangiocarcinoma has received much less attention probably because it is far less common in western countries. Cholangiocarcinoma is relatively uncommon in western countries with the incidence being 0.2-0.7 per 100,000 people (23). On the other hand, the incidence of cholangiocarcinoma in Asian countries is much higher. The incidence of cholangiocarcinoma in Japan and Singapore is 2.8 and 1.0 per 100,000 people, respectively, and the incidence is 7.4 in Korea (24) and 84.6 in northern Thailand. The exceptionally high incidence of cholangiocarcinoma in Thailand and Korea is strongly related with the high prevalence of opisthorchiasis and clonorchiasis.
The evidence for the association between liver fluke infection and bile duct malignancy includes hospital based case-control studies and population-based studies that have correlated the incidence of bile duct cancer with the prevalence of liver fluke infection in various geographic areas.
In a hospital-based case series in Thailand, an unusually high incidence of cholangiocarcinoma was observed on autopsy and on the biopsy materials taken from patients with O. viverrini infection. The ratio between hepatocellular carcinoma and cholangiocarcinoma without opisthorchiasis was 8:1, whereas the ratio was reversed among those with fluke infection (25).
There have been several cross-sectional studies regarding the incidence of cholangiocarcinoma in patients with O. viverrini infection in Thailand (26-32). In a population based study in an endemic area of northern Thailand, the age standardized incidence rates of cholangiocarcinoma in the year 1988 were 89.2 and 35.5 per 100,000 men and women respectively, and these were the highest rates in the world (26). In another survey, based on ultrasonographic findings in a rural area in Khon Kaen, there were 4% (15 of 1807 inhabitants) asymptomatic patients with cholangiocarcinoma (29) (Fig. 6). In another prospective case-controlled study, seven of 551 patients with O. viverrini infection had cholangiocarcinoma (31).
In South Korea, there has been a report revealing a six fold increased incidence of cholangiocarcinoma in the Pusan area (in the southern part of Korea) where the prevalence of C. sinensis infection is significantly higher than the other areas (33). More recently, a case controlled study in the same area revealed that identification of C. sinensis in stool was associated with cholangiocarcinoma with a relative risk of 2.7 (34).
In another hospital based control study in Korea, there was evidence that C. sinensis infection was significantly associated with intra- and extrahepatic cholangiocarcinoma with an odds ratio of 2.272 to 8.615 depending upon the diagnostic tests (17). In patients with cholangiocarcinoma, there was evidence of C. sinensis infection in 50-80%, while in control group, there was evidence of fluke infection in only 31%.
Intraductal papillary neoplasm of the bile duct is known to be a premalignant condition and it is often associated with mucin over-production. There have been a few recent papers revealing that the disease was related with C. sinensis infection. Suh et al. (35) reported that five of 16 (31%) of intraductal papillary tumors of the bile duct were associated with C. sinensis infection. Jang et al. (36) reported that when cholangiocarcinoma was associated with C. sinensis infection, intraductal papillary neoplasm was much more common than the usual adenocarcinoma.
The incidence of cholangiocarcinoma in endemic areas in China and its relation with C. sinensis infection has not been reported on. There have been several reports of cholangiocarcinoma associated with O. felineus infection, but few systematic studies have been conducted.
Several researchers have observed that there were various pathologic stages that seem to be a progression towards cholangiocarcinoma in patients with clonorchiasis. Hou (37) observed that the hyperplastic biliary epithelium may have gradually changed and transformed to cholangiocarcinoma in 28 cases of concomitant clonorchiasis and cholangiocarcinoma. Kim (38) described that hyperplastic mucosa, when exposed to C. sinensis infection, may transform to cholangiocarcinoma through various stages of dysplasia. Once infected with clonorchiasis, chronic persistent inflammation for more than 20-30 years results in eventual carcinomatous transformation. Uncontrolled cell proliferation is the hallmark of malignant neoplasm (39).
There are two premalignant lesions in cholangiocarcinogenesis, that is, biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile duct (40). BilIN refers to the flat or micropapillary growth of atypical biliary epithelium, which is classified as a precursor lesion of cholangiocarcinoma in the World Health Organization's classification of tumors (23). This has been called biliary dysplasia (41, 42). The other premalignant lesion called intraductal papillary neoplasm is defined as prominent intraductal papillary growth of the neoplastic biliary epithelium with a distinct fine fibrovascular core and this is often associated with mucin over-production (40, 42-44). BilIN is known to progress to tubular adenocarcinoma whereas intraductal papillary neoplasm is known to transform to either tubular adenocarcinoma or to mucinous adenocarcinoma (colloid carcinoma) (40-44). While BilIN can be identified only microscopically, the intraductal papillary neoplasm forms a sizable tumor and the tumor is identifiable on macroscopic examination (40). These two precancerous lesions are often encountered on pathologic examination of the patients with clonorchiasis (Fig. 5) and the patients with clonorchiasis associated cholangiocarcinoma (Figs. 6, 7, 8). These facts suggest that clonorchiasis induced chronic persistent inflammation may develop to cholangiocarcinoma, and probably through step-wise evolution via precancerous stages.
Experimental infection with C. sinensis in animals has never induced bile duct tumors (45). On the other hand, experimental infection with liver fluke and combined dimethylnitrosamine treatment has produced cholangiocarcinoma. Lee et al. (46) conducted an animal experiment to induce cholangiocarcinoma in hamsters that were infected with C. sinensis by administrating 0.0015% dimethylnitrosamine, and cholangiocarcinoma developed in eight of 11 hamsters in 11 weeks. On the other hand, no tumor developed in either the C. sinensis infected hamsters or the dimethylnitrosamine treated hamsters.
Regarding the development of cholangiocarcinoma in O. viverrini infection, there have been several studies regarding the enhanced susceptibility to malignancy in infected animals by exposure to dietary dimethylnitrosamine (26). It was shown that experimentally infected hamsters with O. viverrini develop tumors after exposure to dimethylnitrosamine, while the uninfected animals did not. In an animal experiment, Satarug et al. (47) reported that all the hamsters receiving a combination of subcarcinogenic doses of dimethylnitrosamine and infection with O. viverrini developed cholangiocarcinomas, while either dimethylnitrosamine or O. viverrini infection alone did not cause the development of cancer. These studies suggested that dietary nitrosamines initiates the development of cancer. Further studies have suggested that liver fluke infection enhanced the endogenous nitrosamine production. Haswell-Elkins et al. (27) demonstrated elevated levels of salivary nitrate and urinary and plasma nitrate among men with a moderate and heavy infection as compared with that of uninfected controls, and this reflects endogenous generation of nitric oxide from liver fluke infection. Based on these animal experiments, nitrosamines are considered to act as genotoxicants that exert carcinogenic effects, while liver fluke infection is considered to play an epigenetic role (48).
On the basis of the high prevalence of liver fluke infection in certain endemic areas, it is recommended to do a screening test such as a stool ova test. The high risk groups for liver fluke infection are those who had a history of eating a significant amount of raw, freshwater fish in endemic areas and those who have ultrasonographic or CT images showing dilatation of the peripheral intrahepatic bile ducts, or combinations of these findings (17). When people are infected, anthelminthic drug should be prescribed.
Cholangiocarcinoma develops at an older age, namely, at the age over 50. In a hospital based control study in Korea, about 50-80% of the patients with cholangiocarcinoma had evidence of C. sinensis infection (17) and they were aged patients (average age: 64, age range: 32-79 years). Therefore, men and women older than 50 and who had history of clonorchiasis in the past, whether treated or not, should be put in a screening program for cholangiocarcinoma. Also, those people who show unexplained intrahepatic bile duct dilatation on sonography and/or CT images should be screened. The characteristic radiological finding of past/healed liver fluke infection is diffuse dilatation of the peripheral intrahepatic bile ducts without evidence of an obstructing cause (dilatation without obstruction) on ultrasound, CT or MR imaging (5). This image finding is frequently encountered in healthy looking but infected people in endemic areas.
There have been several papers regarding the usefulness of carbohydrate antigen 19-9 (CA19-9) as a marker to screen for cholangiocarcinoma. CA19-9 was useful for predicting cholangiocarcinoma in patients with primary sclerosing cholangitis, with the sensitivity being 79%, the specificity 99%, the adjusted positive predictive value 57% and the negative predictive value 99% (49). In patients without primary sclerosing cholangitis, the sensitivity of CA19-9 for diagnosing cholangiocarcinoma was 53-67% and the specificity was 76-87% (50-52). The usefulness of CA19-9 for diagnosing cholangiocarcinoma in patients with liver fluke infection has not yet been investigated. Also, the use of radiological examinations as a screening tool for making the diagnosis of cholangiocarcinoma is not known. Many patients with cholangiocarcinoma have evidence of C. sinensis infection (17) and this is the rationale for cholangiocarcinoma screening in those people who were once infected with liver flukes. There were 4% asymptomatic patients with cholangiocarcinoma detected on ultrasound screening in a rural area in Khon Kaen (Thailand) (29).
Intraductal papillary neoplasm of the bile duct is a premalignant condition that predominantly exhibits intraductal growth (23). Intraductal papillary neoplasm accounts for about 15% of all cholangiocarcinoma patients and it transforms to tubular adenocarcinoma or mucinous adenocarcinoma at a late stage (40-44). There have been a few papers revealing that this disease was related with C. sinensis infection (35, 36). Therefore, detection and resection of intraductal papillary neoplasm before transformation to malignant tumor may lead to cure of the disease. In this regard, the at-risk groups should be screened and the patients need preemptive surgical resection.
Liver fluke infection is initiated by ingestion of fluke-infested, raw, fresh-water fish and it causes chronic inflammatory bile duct disease. Once infected, the flukes reside in the bile duct for two or three decades. Though mostly dormant, longstanding infection causes unexplainable lassitude and reduces productivity, and it degrades the quality of life. The complications are bile duct stone formation, recurrent pyogenic cholangitis and bile duct cancer. More than 35 million people worldwide are infected. The exceptionally high incidence of cholangiocarcinoma in Thailand and Korea is attributed to the high prevalence of liver fluke infection in these areas. Traditional eating habits of raw fresh-water fish, which is believed to be a health-promoting food, brings about "the evils of health."
The strategy for control of foodborne infection clearly requires collaboration between the sectors of public health, the food industry as well as journalism. Consumer education is crucial and of the utmost importance. In this regard, physicians should know about the high prevalence of liver fluke infection among healthy looking people and the long term consequencies of the infection, and particularly the development of cholangiocarcinoma should be emphasized. Additionally, once it is recognized, the infected person should be monitored for the development of bile duct cancer. Physicians should change their attitude towards liver fluke infection. Liver flukes should not be neglected or abandoned as they are evils of human health.
Figures and Tables
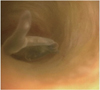 | Fig. 1Adult Clonorchis sinensis within intrahepatic bile duct.
Endoscopy reveals "rayfish-like" or "leech-like" flat worm moving within lumen, and it has whitish-yellow ventral sucker at tapered head portion of worm. Note reddish inner surface of bile duct at background as mark of blood sucking (Courtesy of Dong Wan Seo, MD, Asan Medical Center, Seoul, Korea).
|
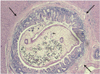 | Fig. 2Photomicrograph of rabbit bile duct that was experimentally infected with C. sinensis, and it shows adult fluke in bile duct. Note extensive epithelial hyperplasia of mucosa around fluke and periductal fibrosis (arrows) (Hematoxylin & Eosin staining, × 40). |
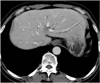 | Fig. 370-year-old man with clonorchiasis and he complained of lassitude.
He had been previously admitted 10 years ago because of jaundice and he was diagnosed with clonorchiasis and treated with praziquantel. Five years later, he had cholecystectomy for gallbladder stones and cholecystitis. Since then, he has been suffering from general weakness and lassitude. He has been eating raw fresh-water fish frequently for more than 12 years because he believed raw fish was helpful for his health. CT shows mild dilatation of small intrahepatic bile ducts up to periphery of liver without dilatation of large bile ducts, which is characteristic for clonorchiasis.
|
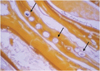 | Fig. 4Photomicrograph of C. sinensis eggs (arrows) in calcium-bilirubinate stone (Hematoxylin & Eosin staining, × 400). (Reprinted from AJR Am J Roentgenol 1991;157:1-8) |
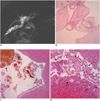 | Fig. 563-year-old man with clonorchiasis and intrahepatic bile duct stones.
A. MR cholangiogram shows severe dilatation of posterior inferior segmental branch of right hepatic lobe and it is filled with multiple stones. There is no definite intraductal mass. Note mild dilatation of the other segmental bile ducts. B-D. Microphotographs show several adult C. sinensis (B), bile duct stone (arrow in C) and low-grade biliary intraepithelial neoplasia (D) (B-D; Hematoxylin & Eosin staining, × 200). Note micropapillary growth of atypical biliary epithelium (arrows in D). Biliary intraepithelial neoplasia is microscopic structure and this is not visible macroscopically or on radiological images.
|
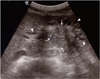 | Fig. 6Cholangiocarcinoma that was incidentally found in patient with opisthorchiasis.
Transverse sonogram of middle age man who was included in sonographic survey in village near Vientiane, Laos shows 3.0 cm mass (arrows) associated with dilatation of bile ducts peripheral to mass (arrowheads). CT image of same patient showed mass, which was consistent with cholangiocarcinoma (not shown).
|
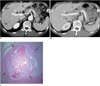 | Fig. 7Intraductal papillary adenocarcinoma that developed during three years of follow-up in patient with clonorchiasis.
A. CT shows mild dilatation of intrahepatic duct (arrows). B. CT image three year later shows focal dilatation of segmental bile duct, which is filled with small, soft tissue mass (arrow). C. Microphotograph shows focally invasive tubular adenocarcinoma that developed from papillary adenoma within lumen of bile duct (arrows). Intraductal papillary neoplasm is sizable and it is visible macroscopically and radiologically (scanning power view, × 1).
|
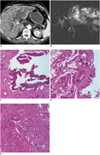 | Fig. 8Cholangiocarcinoma at common hepatic duct in 72-year-old man with severe clonorchiasis.
A. CT shows mass at bifurcation of right and left hepatic ducts (arrow) and severe dilatation of entire intrahepatic duct up to surface of liver. B. MR cholangiogram shows defect at common hepatic duct (arrows), which represents cholangiocarcinoma. C. Microphotograph from common hepatic duct (adjacent to main tumor) shows high grade biliary intraepithelial neoplasia, which is precursor of cholangiocarcinoma (Hematoxylin & Eosin staining, × 200). D. Microphotograph from common hepatic duct (adjacent to main tumor) shows intraductal tubular adenocarcinoma and goblet cell hyperplasia (arrows) (Hematoxylin & Eosin staining, × 200). E. Microphotograph from common hepatic duct (main tumor) shows adenosquamous carcinoma (Hematoxylin & Eosin staining, × 100).
|
References
1. Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1995. 849:1–157.
2. Rim HJ, Kim KH, Joo KH. Classification and host specificity of Metagonimus spp. from Korean freshwater fish. Korean J Parasitol. 1996. 34:7–14.
3. Rim HJ. Clonorchiasis: an update. J Helminthol. 2005. 79:269–281.
4. Jongsuksuntigul P, Imsomboon T, Teerarat S, Suruthanavanith P, Kongpradit S. Consequences of opisthorchiasis control programme in northeastern Thailand. J Trop Med Parasitol. 1996. 19:24–39.
5. Lim JH. Radiologic findings of clonorchiasis. AJR Am J Roentgenol. 1990. 155:1001–1008.
6. Riganti M, Pungpak S, Punpoowong B, Bunnag D, Harinasuta T. Human pathology of Opisthorchis viverrini infection: a comparison of adults and children. Southeast Asian J Trop Med Public Health. 1989. 20:95–100.
7. Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003. 88:209–220.
8. Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003. 88:221–227.
9. Harinasuta T, Riganti M, Bunnag D. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung. 1984. 34:1167–1169.
10. Upatham ES, Viyanant V, Kurathong S, Rojborwonwitaya J, Brockelman WY, Ardsungnoen S, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull World Health Organ. 1984. 62:451–461.
11. Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005. 5:31–41.
12. Coordinating Office of the National Survey on the Important Human Parasitic Diseases. A national survey on current status of the important parasitic diseases in human population. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005. 23:332–340.
13. Jongsuksuntigul P, Imsomboon T. Epidemiology of opisthorchiasis and national control program in Thailand. Southeast Asian J Trop Med Public Health. 1998. 29:327–332.
14. Kaewpitoon N, Kaewpitoon SJ, Pengsaa P. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol. 2008. 14:2297–2302.
15. Upatham ES, Viyanant V, Kurathong S, Brockelman WY, Menaruchi A, Saowakontha S, et al. Morbidity in relation to intensity of infection in Opisthorchiasis viverrini: study of a community in Khon Kaen, Thailand. Am J Trop Med Hyg. 1982. 31:1156–1163.
16. Prevalence of intestinal parasitic infection in Korea, the 7th Report. 2004. Korea Association of Health Promotion;122–123.
17. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006. 44:1066–1073.
18. Flavell DJ. Liver-fluke infection as an aetiological factor in bile-duct carcinoma of man. Trans R Soc Trop Med Hyg. 1981. 75:814–824.
19. Pae WK. Bacteriological and parasitological study of stone. J Korean Surg Soc. 1968. 10:377–380.
20. Joo KR, Bang SJ. A bile based study of Clonorchis sinensis infections in patients with biliary tract diseases in Ulsan, Korea. Yonsei Med J. 2005. 46:794–798.
21. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. J Gastroenterol Hepatol. 2008. 23:e399–e404.
22. Wong WT, Teoh-Chan CH, Huang CT, Cheng FC, Ong GB. The bacteriology of recurrent pyogenic cholangitis and associated diseases. J Hyg (Lond). 1981. 87:407–412.
23. Nakanuma Y, Sripa B, Vantanasapt V, Leong A-Y, Ponchon T, Ishak KG. Hamilton SR, Aaltonen LA, editors. Intrahepatic cholangiocarcinoma. World Health Organization classification of tumors. Pathology and genetics of tumours of the digestive system. 2000. Lyon, France: IARC Press;173–180.
24. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.
25. Bhamarapravati N, Virranuvatti V. Liver diseases in Thailand. An analysis of liver biopsies. Am J Gastroenterol. 1966. 45:267–275.
26. Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002. 89:962–970.
27. Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, et al. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994. 305:241–252.
28. Elkins DB, Mairiang E, Sithithaworn P, Mairiang P, Chaiyakum J, Chamadol N, et al. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996. 55:295–301.
29. Haswell-Elkins MR, Mairiang E, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994. 59:505–509.
30. Sithithaworn P, Haswell-Elkins MR, Mairiang P, Satarug S, Mairiang E, Vatanasapt V, et al. Parasite-associated morbidity: liver fluke infection and bile duct cancer in northeast Thailand. Int J Parasitol. 1994. 24:833–843.
31. Kurathong S, Lerdverasirikul P, Wongpaitoon V, Pramoolsinsap C, Kanjanapitak A, Varavithya W, et al. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985. 89:151–156.
32. Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990. 84:715–719.
33. Chung CS, Lee SK. An epidemiological study of primary liver carcinomas in Pusan area with special reference to clonorchiasis. Korean J Pathol. 1976. 10:33–46.
34. Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996. 25:933–940.
35. Suh KS, Roh HR, Koh YT, Lee KU, Park YH, Kim SW. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology. 2000. 31:12–17.
36. Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008. 453:589–598.
37. Hou PC. The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J Pathol Bacteriol. 1956. 72:239–246.
38. Kim YI. Liver carcinoma and liver fluke infection. Arzneimittelforschung. 1984. 34:1121–1126.
39. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.
40. Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006. 44:350–358.
41. Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000. 7:542–550.
42. Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001. 34:651–658.
43. Nakanuma Y, Sasaki M, Ishikawa A, Tsui W, Chen TC, Huang SF. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002. 17:851–861.
44. Shimonishi T, Zen Y, Chen TC, Chen MF, Jan YY, Yeh TS, et al. Increasing expression of gastrointestinal phenotypes and p53 along with histologic progression of intraductal papillary neoplasia of the liver. Hum Pathol. 2002. 33:503–511.
45. Lee JH, Yang HM, Bak UB, Rim HJ. Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two-step carcinogenesis. Korean J Parasitol. 1994. 32:13–18.
46. Lee JH, Rim HJ, Bak UB. Effect of Clonorchis sinensis infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian golden hamsters. Korean J Parasitol. 1993. 31:21–30.
47. Satarug S, Haswell-Elkins MR, Sithithaworn P, Bartsch H, Ohshima H, Tsuda M, et al. Relationships between the synthesis of N-nitrosodimethylamine and immune responses to chronic infection with the carcinogenic parasite, Opisthorchis viverrini, in men. Carcinogenesis. 1998. 19:485–491.
48. Thamavit W, Pairojkul C, Tiwawech D, Shirai T, Ito N. Strong promoting effect of Opisthorchis viverrini infection on dimethylnitrosamine-initiated hamster liver. Cancer Lett. 1994. 78:121–125.
49. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005. 50:1734–1740.
50. Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000. 95:204–207.
51. John AR, Haghighi KS, Taniere P, Esmat ME, Tan YM, Bramhall SR. Is a raised CA 19-9 level diagnostic for a cholangiocarcinoma in patients with no history of sclerosing cholangitis? Dig Surg. 2006. 23:319–324.
52. Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004. 24:139–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download