Abstract
Lymphangioleiomyomatosis (LAM) is a rare idiopathic disease and this is characterized by a proliferation of abnormal smooth muscle cells in the lungs and in the lymphatic system of the thorax and retroperitoneum. The female genital tract is rarely affected by LAM. We report here on the CT and MR imaging findings of extensive LAM involving the uterus and pelvic cavity, and this was seen as multiple cystic uterine and parauterine masses with internal hemorrhage in a young female with tuberous sclerosis complex.
Lymphangioleiomyomatosis (LAM) is a rare idiopathic disease that exclusively occurs in women of childbearing age, and this disease is characterized by the proliferation of abnormal smooth muscle cells in the lungs and along the thoracic and abdominal lymphatics (1-3). The disease primarily affects the lungs in the majority of cases, but extrapulmonary LAM occasionally occurs with or without subsequent involvement of the lungs. LAM involvement of the uterus is extremely rare and there have been only a few such reported cases (2-7), and the imaging findings of uterine LAM have only been briefly mentioned in two reports (2, 5). In this article, we report the cross-sectional imaging findings of extensive LAM involving the uterus and pelvic cavity in a 29-year-old woman with tuberous sclerosis complex (TSC) and we propose that certain imaging features and the clinical history may suggest the diagnosis. To the best of our knowledge, this is the first report of the imaging findings of LAM involvement of female pelvic organs.
A 29-year-old woman presented with diffuse abdominal distension and chronic anaemia. When she was ten years old, she was diagnosed with TSC based on her history of seizure and mental retardation. She also had hypomelanotic macules in both buttock areas. She had no family history of TSC. Five years previously, she underwent a pelvic magnetic resonance imaging (MRI) study for the evaluation of menometrorrhagia and dysmenorrhea. The MR images showed several small (less than 3 cm) intramural and subserosal hemorrhagic lesions in the uterus and hematometra (Fig. 1A, B). Adenomyotic cysts and uterine leiomyomas with red degeneration were considered in the differential diagnosis based on the imaging at that time. Endometrial drainage was performed for the hematometra, but no further treatment such as hormonal therapy was done.
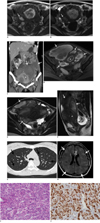
For the current visit, her serum CA-125 level was 204.01 U/ml, the CA 19-9 level was 50.11 U/ml and the hemoglobin level was 8.7 g/dl. The renal function was within the normal range. Contrast-enhanced abdominal computed tomography (CT) (Fig. 1C) showed multiple, large, lobulated, thick-walled cystic masses involving the uterus and the entire pelvic cavity, and these masses extended to the lower abdomen. The attenuation of the cystic masses was higher than that of simple fluid, which suggested hemorrhagic contents. There was no evidence of internal calcification or a solid component. The endometrial cavity was dilated and filled with high density fluid. Both kidneys were enlarged by multiple, various sized masses with attenuations matching fat and soft tissue, which are findings compatible with angiomyolipomas. Pelvic MR imaging showed huge, irregularly-shaped, cystic masses involving the uterus and parauterine pelvic cavity, and these masses were predominantly hyperintense on the fat-saturated T1-weighted image (Fig. 1D) and they were heterogeneously hypointense and hyperintense on the T2-weighted image (Fig. 1E). These cystic masses were thought to originate from the uterine myometrium on the sagittal T2-weighted image (Fig. 1F). The uterine cavity was distended with hematoma. Multiple loculated fluid collections with fluid-fluid levels were seen in the cul-de-sac. On the contrast-enhanced T1-weighted image, there was no enhancing solid portion in the masses. Markedly enlarged LAM involving the uterus with extension to the pelvic cavity was suggested as the most probable diagnosis based on the follow-up imaging and clinical findings. The chest CT showed numerous, well-defined, thin-walled cysts that were diffusely distributed throughout the lungs (Fig. 1G). Because the cysts were regularly round in shape and there was no associated nodular lesion, pulmonary LAM was the suggested diagnosis, but she had no pulmonary symptoms. Brain MR imaging showed multiple cortical and subcortical tubers as well as several subependymal nodules in the bilateral lateral ventricles, and this represented tuberous sclerosis (Fig. 1H).
Total hysterectomy and bilateral adnexectomy with adhesiolysis were performed. The intraoperative findings revealed that the uterus was enlarged and distorted by multiple subserosal and intramural hemorrhagic cystic masses. The cystic masses also involved both adnexae, the pelvic side wall and the omentum. There were severe adhesive changes between the cystic lesions and the sigmoid colon and bladder. Microscopic examination revealed that the tumor was composed of atypical smooth muscle cells (LAM cells) arranged in short fascicles around dilated lymphatics and a ramifying network of endothelium-lined spaces (Fig. 1I). Immunohistochemical staining showed that the tumor cells were diffusely positive for smooth muscle actin and they were strongly multifocally positive for human melanin black-45 (HMB-45) (Fig. 1J). The above histological and immunohistochemical findings were consistent with LAM. The serosal layers of the right ovary and left fallopian tube had LAM involvement. The patient's hospital course was uneventful and she was discharged on the tenth postoperative day.
Lymphangioleiomyomatosis is a rare idiopathic disease that is found almost exclusively in premenopausal women. LAM results from the proliferation of abnormal-appearing smooth muscle cells in the lymph vessels, which causes dilatation and obstruction in the lymph vessels and this results in cystic collections of chylous material (1). These smooth muscle cells are classified in the family of perivascular epithelioid cells (PEC), which is a cell type that is constantly present in a group of tumors, including LAM, sugar tumors of the lung and pancreas, renal angiomyolipomas and clear cell myomelanocytic tumor of the falciform ligament (8, 9). These so-called "PEComa" all express HMB-45 (8). LAM occurs in about 30% of the women with tuberous sclerosis complex (TSC), which is an autosomal dominant multisystem neurocutaneous disorder of highly variable penetrance, and it is characterized by hamartomas, seizure and mental retardation (1). Due to the striking similarities in the pathological processes between the LAM and TSC, LAM has been considered a forme fruste of TSC, and LAM is classified as a TSC-associated or sporadic form (5, 10).
Lymphangioleiomyomatosis predominantly affects the lung parenchyma and this is characterized by pulmonary cysts seen on CT. The extrapulmonary manifestation of LAM is uncommon and this is mainly located in the retroperitoneum, pelvic cavity and the posterior mediastinum along the lymphatic channels (1-7). In a large study of 80 patients with pulmonary LAM (11), the CT imaging findings of retroperitoneal LAM were described as a low-attenuating (3-25 Hounsfield unit [HU]), multilobulated mass, and the ultrasound findings were reported as a cystic mass with a thick echogenic rind.
Uterine involvement of LAM is extremely rare, and to the best of our knowledge, only eight cases of pathologically proven uterine LAM have currently been reported (2-7). Six cases of uterine LAM occurred in patients with the stigmata of TSC, and two patients did not have stigmata of TSC. Most uterine LAMs were microscopic and they are incidentally found in patients undergoing evaluation for extrauterine disease (2). Menorrhagia and/or pelvic pain have been reported in half of the cases, the same as in our case (2-7). The imaging findings of uterine LAM were briefly mentioned in two gynaecological reports (2, 5). Longacre et al. (2) reported that uterine LAM simulated high-stage endometrial stromal sarcoma, and the CT finding of uterine LAM was described as a large uterine mass, which was thought to be a fibroid. However, the surgery revealed multiple subserosal and intramural hemorrhagic nodules ranging in size from 2.5 to 4.0 cm and bloody ascites due to tumor perforation. Han et al. (5) recently reported on a 5.5-cm hypervascular tumor between the uterus and the right ovary that showed low signal intensity on the T1-weighted MR image and intermediate signal intensity on the T2-weighted MR image. This MRI finding is quite different from that of retroperitoneal LAM and it is rather similar to that of uterine leiomyoma. The pathological examination in that case revealed multiple intramural leiomyomas and several fragments of irregular soft masses composed of HMB 45 positive LAM cells.
The most remarkable aspects of our case are the initial involvement of LAM in the uterus that gradually grew into the pelvic cavity and the intratumoral bleeding. The clinical presentation was menometrorrhagia and dysmenorrhea (i.e., not pulmonary symptoms). Intratumoral bleeding may be caused by overdistention and rupture of the cysts. Although the CT findings showed highly attenuating cystic masses involving the uterus and pelvic cavity, the MR imaging confirmed the hemorrhagic cystic nature of the masses by their signal intensity and the MR imaging exactly localized the masses. These imaging findings are consistent with the known imaging findings of retroperitoneal LAM. However, if a patient is without a clinical history of TSC, then uterine intramural and subserosal leiomyomas with hemorrhagic and cystic degeneration, adenomyotic cysts and/or endometrial cysts, and malignant uterine and/or ovarian tumors with hemorrhage should be considered in the differential diagnosis.
In summary, we report here on the extraordinary radiological findings of LAM involving the uterus and pelvic cavity in a young woman with TSC. Although uterine LAM is rare, radiologists should consider the possibility of this disease when they see multiple cystic uterine or parauterine masses with or without internal hemorrhage in a patient with a history of TSC or pulmonary LAM.
Figures and Tables
Fig. 1
MR and CT images of 29-year-old woman with clinical history of tuberous sclerosis complex.
A. Axial T1-weighted MR image shows small hyperintense intramural lesion (arrow) at right side of uterine body. Uterine cavity is markedly distended and filled with high signal intensity fluid (H), which is suggestive of hematometra. Another hyperintense round lesion is seen at right ovary (open arrow). B. Axial T2-weighted MR image shows ovoid intramural lesion of mild hyperintensity (arrow), which is suggestive of intralesional hemorrhage. Right ovarian lesion shows signal shading, which is suggestive of chronic repetitive bleeding in lesion (open arrow). C. Coronal reformatted contrast-enhanced CT obtained five years after initial MR imaging shows multiple, large, lobulated thick-walled high density cystic masses (arrows) involving uterus and pelvic cavity. Endometrial cavity (asterisk) is dilated and filled with high density fluid. Right kidney (RK) is enlarged by multiple, variable sized masses of fat and soft tissue attenuation, and this is compatible with angiomyolipomas. D, E. Axial fat-suppressed T1-weighted image (D) and T2-weighted image (E) show huge, irregularly shaped masses (arrows) involving uterus and parauterine pelvic cavity; there is high signal intensity on T1-weighted image and mixed low and high signal intensity on T2-weighted image. High signal intensity is seen in dilated uterine cavity (asterisk) on T1-weighted image, which suggests hematometra. Multiloculated fluid collections with fluid-fluid levels are seen in cul-de-sac (open arrows). F. Sagittal T2-weighted image shows large, lobulated mass (arrows) that originated from uterine myometrium. Uterine cavity is dilated (asterisk). Small amount of fluid collection is seen in cul-de-sac (open arrow). LO = left ovary. G. Axial chest CT shows numerous, well-defined, thin-walled cysts (arrows), distributed diffusely throughout lungs. H. Axial FLAIR (fluid attenuated inversion recovery) image of brain shows multiple cortical and subcortical tubers (white arrows), as well as subependymal nodule (open arrow), in left lateral ventricle, and this all represents tuberous sclerosis. I. Tumor is composed of smooth muscle cells that are arranged in short fascicles around dilated lymphatic vessels, and this is consistent with lymphangioleiomyomatosis (Hematoxylin & Eosin, × 200). J. Tumor cells show diffuse cytoplasmic staining for HMB-45 (immunostains, × 400).
References
1. Avila NA, Dwyer AJ, Rabel A, Moss J. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: comparison of CT features. Radiology. 2007. 242:277–285.
2. Longacre TA, Hendrickson MR, Kapp DS, Teng NN. Lymphangioleiomyomatosis of the uterus simulating high-stage endometrial stromal sarcoma. Gynecol Oncol. 1996. 63:404–410.
3. Torres VE, Bjornsson J, King BF, Kumar R, Zincke H, Edell ES, et al. Extrapulmonary lymphangioleiomyomatosis and lymphangiomatous cysts in tuberous sclerosis complex. Mayo Clin Proc. 1995. 70:641–648.
4. Gyure KA, Hart WR, Kennedy AW. Lymphangiomyomatosis of the uterus associated with tuberous sclerosis and malignant neoplasia of the female genital tract: a report of two cases. Int J Gynecol Pathol. 1995. 14:344–351.
5. Han JM, Lee KH, Kim SJ, Rhim CC, Park YH, Kang JB, et al. A case of lymphangioleiomyomatosis originated in the pelvic cavity. J Gynecol Oncol. 2008. 19:195–198.
6. Lack EE, Dolan MF, Finisio J, Grover G, Singh M, Triche TJ. Pulmonary and extrapulmonary lymphangioleiomyomatosis. Report of a case with bilateral renal angiomyolipomas, multifocal lymphangioleiomyomatosis, and a glial polyp of the endocervix. Am J Surg Pathol. 1986. 10:650–657.
7. Maziak DE, Kesten S, Rappaport DC, Maurer J. Extrathoracic angiomyolipomas in lymphangioleiomyomatosis. Eur Respir J. 1996. 9:402–405.
8. Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, et al. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996. 20:722–730.
9. Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008. 452:119–132.
10. McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008. 133:507–516.
11. Avila NA, Kelly JA, Chu SC, Dwyer AJ, Moss J. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000. 216:147–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download