Abstract
Tumor-mimicking lesions in the musculoskeletal system can be defined as lesions mistaken as tumors due to the presence of palpation upon physical examination or a tumor-like appearance upon radiological examination. Moreover, tumor-mimicking lesions show diverse etiologies and anatomic locations. We illustrated the various tumor-mimicking lesions involving bone and soft tissue. In this review, the tumor-mimicking lesions were classified into those based on clinical examination and those based on radiological examination in musculoskeletal radiology. Awareness of the various causes of tumor-mimicking lesions, correctly obtaining clinical information, and the proper selection of imaging modality are important for the differentiation of tumor-mimicking lesions from true neoplasms.
Tumor-mimicking lesions in the musculoskeletal system can be defined as lesions mistaken as tumors or neoplasms due to a palpable sensation upon physical examination or a tumor-like appearance upon radiological examination. In clinical practice, it is not rare for radiologists to encounter the tumor-mimicking lesions in the musculoskeletal system in addition to an initial misdiagnosis of it as a tumor. The etiology and anatomic locations of tumor-mimicking lesions are so variable, ranging from normal variants or pseudolesions to traumatic or inflammatory lesions. Moreover, these lesions have been sporadically reported (1-6).
In this study, we categorized the tumor-mimicking lesions in the musculoskeletal system according to the conditions that raised suspicion for the presence of tumors or neoplasms as follows; the lesions manifesting as palpable masses upon physical examination are categorized as tumor-mimicking lesions based on clinical examination. On the other hand, the lesions which represent radiological features indicative of neoplasms on initial or any solitary imaging modality, but are finally diagnosed as non-neoplastic lesions by another imaging modalities, are categorized as a tumor-mimicking lesions based on a radiological assessment.
We present the various tumor-mimicking lesions involving bone and soft tissue, and provide guidance for a diagnostic approach in order to minimize the misdiagnosis and to solve the causes.
Most parts of the human body have a symmetric appearance. If there is a discrepancy in size, course, or orientation between sides (Fig. 1), there may be a subjective impression of the presence of a mass lesion.
Normal variants, such as accessory bones, can also manifest as hard, palpable lesions in the hands or feet, with or without pain (7, 8), including the os acromiale, os externum tibiale, styloid bone, and sesamoid bones. Accessory bones are usually symmetric but can be unilateral. CT and MRI are effective at differentiating tumor-mimicking lesions from other osseous neoplasms such as osteochondromas that show continuity in cortical and medullary bones. Use of markers and bilateral imaging for comparison may help in obtaining an accurate diagnosis.
Hypertrophy of a tendon or muscle may result in the presence of a palpable mass-like lesion with mild pain (7, 9). Findings such as thickening of a tendon, with or without muscle contraction in the absence of evidence of an adjacent mass lesion, allow for the correct diagnoses of these entities (Figs. 2, 3). These entities may be associated with a large region of altered marrow signal intensity, a cortical focal defect, and altered adjacent soft tissue at the tendon insertion site. Muscle hypertrophy can develop in any location and is related to overuse, excessive physical activity, cervical dystonia, nerve lesions, and idiopathic causes (Fig. 4).
Supernumerary or anomalous muscles in the forearms and hands are relatively common (1, 3, 4). These muscles can mimic pathologic tumors and may result in symptoms of nerve entrapment if the muscles are located in the carpal tunnel or in Guyon's canal (4). Upon MR imaging, supernumerary or anomalous muscles, show homogeneous signal intensity similar to that of normal skeletal muscle. Awareness of the typical locations and identification of action potentials with electromyography of the palpable lesions can be helpful (1, 4).
Localized prominent fat tissue occasionally develops from rapid changes in weight or from steroid overuse (7). In addition, as part of the aging process, fat tissue has a decrease in consistency, decreased fibrous strands interspersed between fat lobules, and increased mobility of superficial fat tissues, all of which may lead to misconception of a soft tissue tumor.
A plain radiograph may show hypodensity with a bulging configuration or displacement of the adjacent soft tissue or fascial plane. Sonography demonstrates abundant fat deposition with isoechogenicity or slightly increased echogenicity in the subcutaneous layer of the involved site compared to that of the normal adjacent area. Abundant localized fat deposition with less fibrotic strands can be easily detected with MR and CT imaging. Both a weak mass effect and signal intensity similar to that of the adjacent fat, occasionally result in these lesions being missed on imaging. In this situation, a marker or bilateral imaging may be useful (Fig. 5).
Superficial thrombophlebitis is an inflammation of a superficial vein resulting from a blood clot and may occur spontaneously or as a complication of intervention (10). Symptoms including pain, swelling, or redness can be associated with thrombophlebitis; whereas, hardening and a cord-like palpable mass can be confused with a superficial soft tissue mass lesion. Superficial thrombophlebitis can be easily diagnosed by sonography, and the typical finding is characterized by an echogenic cord-like structure that has a connection with veins, with no collapse on compression (Fig. 6).
Crystal deposition disease, such as calcific tendinitis and gouty tophi, can present as a palpable lesion with joint pain and can be misdiagnosed as a bone tumor. Calcific tendinitis is caused by the deposition of calcium hydroxyapatite crystals in tendons (11) (Fig. 8), while gouty arthritis is caused by the deposition of monosodium urate (12). Characteristic findings on radiography and US, in combination with clinical and laboratory data, are diagnostic for these disorders. The actual role of magnetic resonance imaging seems limited to the exclusion of neoplasms and better demonstration of inflammatory changes in the early stages of the disease.
Granulomas resulting from injection or foreign bodies, can occasionally mimic neoplasms upon physical examination and imaging (13, 14). Radiography shows streaky and nodular calcifications of soft tissue with or without mass formation. Upon sonography, an irregular calcific mass with posterior shadowing can be observed.
Commonly encountered pseudolesions that may give the impression of true osteolytic or cystic lesions on plain radiographs include the superolateral humeral head, rhomboid fossa of the clavicle, scapular defects, the supratrochlear foramen, and an anterior lytic defect of the calcaneus (15).
Superimposition of normal structures can result in increased density on a specific radiographic view that may simulate a pathological lesion. Occasionally, a positioning, in which the scapula projects over a vertebral body, results in an increased opacity that simulates sclerosis on a lateral chest radiograph (16). Correlations with different views may be sufficient to confirm a normal finding (Fig. 10).
Tumor-mimicking lesions manifesting as osteolytic lesions on plain radiographs are mainly attributable to focal fat deposition in marrow or localized osteoporosis. Localized osteoporosis results from the immobilization and disuse followed by paralysis or a previous fracture (7) (Fig. 11). These radiographic findings may result in a misdiagnosis as osseous tumors, such as a simple bone cyst, giant cell tumor, plasma cell tumor, or metastasis. Therefore, CT or MR imaging can be used for the differential diagnosis; both a fat deposition in marrow and localized osteoporosis demonstrate the non-visualization of the soft tissue component and are unlikely to be true osseous tumors.
Soft tissue hematomas may be associated with a traumatic event or minimal trauma in patients with bleeding tendencies such as hemophilia (Fig. 12). In hemophiliacs, it is not rare to present with an intraosseous hematoma mimicking a bone tumor.
In soft tissue hematomas, it may sometimes be challenging to distinguish a simple hematoma from a hemorrhage into a preexisting malignant mass or associated secondary infection. Various patterns of sonographic, CT, and MR images represent different stages of the hemorrhagic event (Fig. 12). The presence of hemoglobin and associated degradation products influence the MRI signal intensities and evolve in a somewhat predictable fashion over time (7).
Myositis ossificans is a common tumor-mimicking lesion that occasionally occurs in immobilized patients after physical therapy or in patients with a history of trauma. A sharply circumscribed bony mass is usually apparent by 6-8 weeks, becomes smaller and mature by 5-6 months, and may show diffuse ossification (17, 18) (Fig. 13B). Early lesions demonstrate an inhomogeneous mass with a high signal intensity on MR T2-weighted images (Fig. 13A). The lesions are seen as isointense relative to muscle on T1-weighted images, and may be accompanied by soft tissue edema (18). Intermediate or older lesions typically demonstrate a rim of curvilinear decreased signal intensity corresponding to peripheral ossification of the lesions. Mature lesions are well-defined homogeneous masses with a signal intensity approximating that of fat on both T1-weighted and T2-weighted images and are present without associated edema. Active lesions demonstrate enhancement as seen on enhanced MR images (18, 19). Without a history of trauma, it may be difficult to differentiate a lesion from a malignant tumor such as an osteosarcoma.
Focal myositis can manifest as a localized intramuscular soft tissue mass that may rapidly increase in size over a period of weeks (20, 21). CT imaging features are nonspecific, with the lesions demonstrating attenuation less than or similar to that of the adjacent muscle. On MR imaging, focal myositis may have a heterogeneous appearance with increased signal intensity and surrounding edema on T2-weighted images (Fig. 14). Sonography shows an increase in the diameter of the affected muscle group and a diffuse increase in the echogenicity of the muscle in the early stages of myositis (10). Relative preservation of muscle fibers is suggestive of myositis rather than a tumorous lesion (20).
Osteomyelitis demonstrates moth-eaten or permeative bone destruction and can frequently mimic a malignant bone tumor, such as metastasis (22) (Fig. 15), multiple myeloma, or Ewing's sarcoma. Chronic osteoarthritis may show extensive bone destruction with extension to the adjacent soft tissue. Without a clinical history or information from previous radiographs, osteomyelitis might be mistaken as a tumor with soft tissue mass formation (Fig. 16).
Hyperparathyroidism can accompany brown tumors. Brown tumors of the bone are not true neoplasms; rather, they are unusual, diffuse, reactive lesions caused by hyperparathyroidism; with the radiological features of osteolytic bone lesions resembling benign tumorous lesions such as simple bone cysts (Fig. 17). Other typical findings of primary or secondary hyperparathyroidism, including subperiosteal bone resorption, osteosclerosis, soft tissue calcifications, and osteoporosis, are commonly associated. Appropriate clinical history and laboratory data are also helpful in the diagnosis.
Amyloidosis can present in a variety of ways at different anatomic sites. Skeletal amyloidosis can manifest as an aggressive, expansile lesion with mixed sclerotic and lytic bony lesions that often simulate neoplasms (23); these are commonly encountered in the hip and shoulder.
Insufficiency fractures can show an ill-defined lytic appearance on radiographs and may mimic bony metastasis (7) (Fig. 18). A background of decreased bone density, other insufficient fractures with the typical appearance of irregular sclerotic lines at typical locations, and evident fracture lines visualized on CT or MRI are helpful in correctly making this diagnosis.
Postoperative complications such as osteolysis may result in a tumor-mimicking lesion, which can cause a well-defined osteolytic lesion with an adjacent soft tissue mass via cortical bone disruption (Fig. 19). Radiographs may underestimate the extent and location of the osteolysis compared with MR or CT imaging. MRI and CT are effective for the evaluation of the surrounding soft tissue envelope and intracapsular synovial deposits. They are also more effective than radiographs for the detection and evaluation of osteolysis, thus aiding in clinical management (24). Differential points of these occurrences from neoplasms are multifocal involvement along the prosthesis and preservation of fat plane in deep soft tissue. Metallosis, which presents as an osteolytic lesion accompanied by faint increased densities, is often associated with significant osteolysis.
In conclusions, we described a wide variety of tumor-mimicking lesions involving the musculoskeletal system. Awareness of the various conditions responsible for tumor-mimicking lesions should help in minimizing unnecessary examinations and patient concerns, making the correct diagnosis, and carrying out the proper treatment. Correlation with clinical history, such as underlying diseases, age, trauma history, surgery, or procedures, is mandatory for an accurate diagnosis. The proper selection of an imaging modality is important in differentiating tumor-mimicking lesions from true neoplasms. Sometimes, a solitary imaging study may misdiagnose a non-tumorous lesion as a tumor. Sonography is a noninvasive method for patients with clinically palpable lesions and can be performed rapidly at a relatively low cost. Meanwhile, CT and MR imaging can provide more information about the components of a lesion in relation to adjacent structures. In addition, use of a marker attached to a suspected site and scanning of the opposite side can be very useful in corroborating the diagnosis.
Figures and Tables
Fig. 1
51-year-old male with right back mass.
A. Transverse sonograms of both scapulas show protruding medial inferior border of right scapula (arrow). B. On axial CT image, right scapula reveals larger size, more superficial location of medial inferior border (arrow), and different course compared to those of contralateral side.
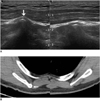
Fig. 2
49-year-old male with painful mass in posterior aspect of right thigh.
Transverse and longitudinal sonograms reveal thickened musculotendinous transition zone of right semitendinosus muscle tendon (arrows) compared to that of contralateral side.
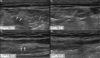
Fig. 3
18-year-old female with palpable lesion on left buttock.
A. There is no space-occupying lesion or signal abnormality near marker (arrow) indicating palpable site, but thickened gluteus maximus tendon at femoral attachment site (star) with slight fatty atrophy of gluteus maximus muscle and depressed groove is noted on axial T1-weighted image. B. Additional axial T1-weighted image of both hips demonstrates prominent thickening of left gluteus maximus tendon (arrow) compared to right side, possibly related to contracture of gluteus muscle.
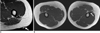
Fig. 4
31-year-old male with palpable mass in left shoulder.
T1-weighted coronal image demonstrates hypertrophy of left trapezius and supraspinatus muscles. Differential diagnosis includes cervical dystonia and accessory nerve injury.
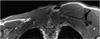
Fig. 5
24-year-old male with focal enlargement of right buttock.
A. Sonograms depict focal increased thickness of subcutaneous fat layer of right buttock (double-headed arrows) that can be compared with contralateral subcutaneous fat layer of left buttock. B. Axial T1-weighted image shows abundant fat deposition without signal change or mass effect in subcutaneous layer of right lateral buttock (arrows). Patient had recent history of rapid weight gain.
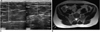
Fig. 6
78-year-old male with cord-like feeling in right forearm.
Segmental obstruction with echogenic materials and dilation of basilic vein with wall thickening, is evident on longitudinal and transverse sonograms, suggesting superficial thrombophlebitis. Patient had history of intravenous catheterization in right forearm.

Fig. 7
65-year-old male with pulsating palpable mass in right inguinal region.
A. Color Doppler sonogram shows blood flow in pseudoaneurysm cavity with characteristic swirling appearance and communicating channel with femoral artery at its base. B. Angiography reveals large pseudoaneurysm (arrow) in right superficial femoral artery.
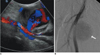
Fig. 8
57-year-old male with painful nodule on lateral aspect of left thigh.
A. Longitudinal sonogram shows calcified nodule with obvious acoustic shadowing in lateral aspect of proximal third of left femur. B. Radiograph of left femur shows round, homogeneous, cloud-like collection of calcifications at lateral aspect of left femur (arrow).
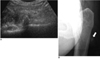
Fig. 9
66-year-old male with migrating palpable lesions in right thigh.
Few well-defined, tortuous, lobulating, hypoechoic, tubular lesions in subcutaneous fat layer of right thigh are demonstrated on sonograms. Note thin linear hyperechogenicity (arrow) in hypoechoic portion of tubular lesions and surrounding vascularity. These were confirmed as sparganosis infestation.

Fig. 10
54-year-old female with neck pain.
A. Lateral view of cervical spine shows small, round sclerotic lesion with radiolucent rim (arrow) mimicking osteoid osteoma. B. As seen on follow-up lateral and right oblique views, there is no corresponding lesion in posterior element of C2. MRI confirmed that this was pseudolesion, which was thought to be superimposition of normal structures on lateral view.
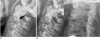
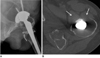
Fig. 11
45-year-old male with history of scaphoid fracture.
A. Ill-defined osteolytic lesion of distal radius (arrows) is depicted on plain radiograph. B. Axial CT scan reveals localized fat deposition in corresponding site on distal radius. Absence of capsule is feature that differentiates this from intraosseous lipoma.
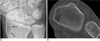
Fig. 12
44-year-old hemophiliac with swelling of right thigh.
A. Plain radiograph shows soft tissue mass with extrinsic bony erosion and periosteal reaction at right femur. B. Sagittal T2-weighted image demonstrates multilobular cystic lesion with dark-signal peripheral nodules, suggesting hemosiderin deposits. Diagnosis is intramuscular and subperiosteal hemophilic pseudotumor involving right thigh.
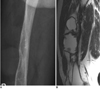
Fig. 13
38-year-old male with history of trauma.
A. Coronal T2-weighted image shows marked heterogeneous lesion with high signal intensity in medial aspect of right thigh, resembling soft tissue sarcoma. B. Plain radiograph taken two months later demonstrates irregular calcification located along muscle on medial aspect of right thigh. Diagnosis was myositis ossificans.
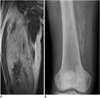
Fig. 14
39-year-old female with palpable mass in her medial thigh.
Fat-suppressed axial T2-weighted image shows heterogeneous soft tissue mass located in distal part of right vastus medialis muscle, mimicking soft tissue sarcoma. Fat-suppressed enhanced T1-weighted image demonstrates mass with strong peripheral enhancement and central necrosis. This mass was histologically confirmed as proliferative myositis.
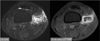
Fig. 15
61-year-old female with history of complete remission of acute leukemia.
A. Multiple peripherally-enhancing nodular lesions involving thoracolumbar vertebrae are noted on fat-suppressed enhanced T1-weighted sagittal MR image, which mimic metastases. B. Portal phase of abdomen CT shows multiple low-attenuated lesions in liver and spleen, which were confirmed as candidial infection. Decompression of epidural mass at T10 was performed, and spinal lesions were also diagnosed as candidiasis.
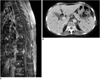
Fig. 16
47-year-old male with left hip pain.
A. Plain radiograph demonstrates expansile, lobulating, osteolytic lesion in left ilium. Initial diagnosis was chondromyxoid fibroma. B. Coronal T2-weighted MR image shows extensive heterogeneous soft tissue mass (star) involving left ilium and sacrum with associated fistula (arrow), which was pathologically confirmed as chronic osteomyelitis.
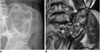
Fig. 17
66-year-old female with complaints of right shoulder pain.
A. Well-defined osteolytic lesion in right proximal humerus is noted on radiograph, suggesting benign tumor. B. Subperiosteal resorption is observed on phalanx. Parathyroid scan with Tc-99m MIBI, not shown, disclosed huge parathyroid mass. Therefore, osteolytic lesion was diagnosed as brown tumor caused by hyperparathyroidism.
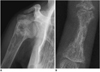
Fig. 18
63-year-old female with recent pain in pelvis.
A. Plain radiograph of pelvis shows osteolytic lesion (arrow) of left pubic bone, mimicking metastasis. B. Two coronal T2-weighted images demonstrate fluid collection at left pubic bone, suggesting insufficiency fracture. Note another insufficiency fracture involving both sacral wings (open arrows).
References
1. Vessal S, Rai SB. Accessory extensor carpi radialis brevis muscle, a pseudomass of the distal forearm: ultrasound and MR appearances - case report and literature review. Clin Radiol. 2006. 61:442–445.
2. Mattle HP, Hess CW, Ludin HP, Mumenthaler M. Isolated muscle hypertrophy as a sign of radicular or peripheral nerve injury. J Neurol Neurosurg Psychiatry. 1991. 54:325–329.
3. Polesuk BS, Helms CA. Hypertrophied palmaris longus muscle, a pseudomass of the forearm: MR appearance--case report and review of the literature. Radiology. 1998. 207:361–362.
4. Anderson MW, Benedetti P, Walter J, Steinberg DR. MR appearance of the extensor digitorum manus brevis muscle: a pseudotumor of the hand. AJR Am J Roentgenol. 1995. 164:1477–1479.
5. Capelastegui A, Astigarraga E, Fernandez-Canton G, Saralegui I, Larena JA, Merino A. Masses and pseudomasses of the hand and wrist: MR findings in 134 cases. Skeletal Radiol. 1999. 28:498–507.
6. Kalbermatten DF, Kalbermatten NT, Hertel R. Cotton-induced pseudotumor of the femur. Skeletal Radiol. 2001. 30:415–417.
7. Resnick D. Diagnosis of bone and joint disorders. 1995. 3rd ed. Philadelphia: Saunders.
8. Mellado JM, Ramos A, Salvadó E, Camins A, Danús M, Saurí A. Accessory ossicles and sesamoid bones of the ankle and foot: imaging findings, clinical significance and differential diagnosis. Eur Radiol. 2003. 13:Suppl 6. L164–L177.
9. Anderson SE, Johnston JO, Steinbach LS. Pseudotumors of the shoulder invited review. Eur J Radiol. 2008. 68:147–158.
10. Useche JN, de Castro AM, Galvis GE, Mantilla RA, Ariza A. Use of US in the evaluation of patients with symptoms of deep venous thrombosis of the lower extremities. Radiographics. 2008. 28:1785–1797.
11. Ramon FA, Degryse HR, De Schepper AM, Van Marck EA. Calcific tendinitis of the vastus lateralis muscle. A report of three cases. Skeletal Radiol. 1991. 20:21–23.
12. Thiele RG, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford). 2007. 46:1116–1121.
13. Schwartzfarb EM, Hametti JM, Romanelli P, Ricotti C. Foreign body granuloma formation secondary to silicone injection. Dermatol Online J. 2008. 14:20.
14. Catalano OA, Dal Pozzo F, Grifi DN, Menchi I, Rosenthal DI. Paraffinoma of the knee. Skeletal Radiol. 2003. 32:485–488.
15. De Wilde V, De Maeseneer M, Lenchik L, Van Roy P, Beeckman P, Osteaux M. Normal osseous variants presenting as cystic or lucent areas on radiography and CT imaging: a pictorial overview. Eur J Radiol. 2004. 51:77–84.
16. Hammond I, Sheikh A, Rasuli P, Souza CA. Vertebral pseudolesion on lateral chest radiograph. AJR Am J Roentgenol. 2008. 190:W240–W241.
17. Kransdorf MJ, Meis JM. From the archives of the AFIP. Extraskeletal osseous and cartilaginous tumors of the extremities. Radiographics. 1993. 13:853–884.
18. De Smet AA, Norris MA, Fisher DR. Magnetic resonance imaging of myositis ossificans: analysis of seven cases. Skeletal Radiol. 1992. 21:503–507.
19. Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1991. 157:1243–1248.
20. Gaeta M, Mazziotti S, Minutoli F, Genitori A, Toscano A, Rodolico C, et al. MR imaging findings of focal myositis: a pseudotumour that may mimic muscle neoplasm. Skeletal Radiol. 2009. 38:571–578.
21. Kransdorf MJ, Temple HT, Sweet DE. Focal myositis. Skeletal Radiol. 1998. 27:283–287.
22. Son JM, Jee WH, Jung CK, Kim SI, Ha KY. Aspergillus spondylitis involving the cervico-thoraco-lumbar spine in an immunocompromised patient: a case report. Korean J Radiol. 2007. 8:448–451.
23. Urban BA, Fishman EK, Goldman SM, Scott WW Jr, Jones B, Humphrey RL, et al. CT evaluation of amyloidosis: spectrum of disease. Radiographics. 1993. 13:1295–1308.
24. Park JS, Ryu KN, Hong HP, Park YK, Chun YS, Yoo MC. Focal osteolysis in total hip replacement: CT findings. Skeletal Radiol. 2004. 33:632–640.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download