Abstract
Objective
This study was performed to evaluate the potential clinical value of concurrent chemotherapy and pulsed high intensity focused ultrasound (HIFU) therapy (CCHT), as well as the safety of pulsed HIFU, for the treatment of unresectable pancreatic cancer.
Materials and Methods
Twelve patients were treated with HIFU from October 2008 to May 2010, and three of them underwent CCHT as the main treatment (the CCHT group). The overall survival (OS), the time to tumor progression (TTP), the complications and the current performance status in the CCHT and non-CCHT groups were analyzed. Nine patients in the non-CCHT group were evaluated to determine why CCHT could not be performed more than twice.
Results
The OS of the three patients in the CCHT group was 26.0, 21.6 and 10.8 months, respectively, from the time of diagnosis. Two of them were alive at the time of preparing this manuscript with an excellent performance status, and one of them underwent a surgical resection one year after the initiation of CCHT. The TTP of the three patients in the CCHT group was 13.4, 11.5 and 9.9 months, respectively. The median OS and TTP of the non-CCHT group were 10.3 months and 4.4 months, respectively. The main reasons why the nine patients of the non-CCHT group failed to undergo CCHT more than twice were as follows: pancreatitis (n = 1), intolerance of the pain during treatment (n = 4), palliative use of HIFU for pain relief (n = 1) and a poor physical condition due to disease progression (n = 3). No major complications were encountered except one case of pancreatitis.
Pancreatic cancer has a dismal prognosis, and most cases of pancreatic cancer are unresectable at the time of diagnosis because pancreatic cancer tends to involve the major vital vessels even in the early stages. Therefore, many pancreatic cancer patients have no choice other than to depend on chemotherapy, even when there is no evidence of distant metastasis at the time of diagnosis. In this regard, many studies have focused on developing more effective chemotherapeutic drugs and enhancing drug delivery to the cancers, or they have examined the effects of adding locoregional therapy.
High intensity focused ultrasound (HIFU) has emerged in recent years as a new noninvasive treatment for solid malignant tumors, including pancreatic cancer (1). An early clinical study concluded that HIFU was safe and feasible for the treatment of advanced pancreatic cancer with a median overall survival (OS) of 11.25 months (range: 2-17 months) (2). Their results suggested that HIFU offers survival benefits given that the median OSs of gemcitabine and of gemcitabine-capecitabine chemotherapies are 6-10 months for patients with advanced pancreatic cancer (3-6). However, they treated patients using a continuous (not pulsed) HIFU machine with a very high acoustic intensity of 5-20 kW/cm2, which required general anesthesia or spinal anesthesia because these intensities delivered as a continuous beam cause intolerable pain and severe injury to the adjacent organs due to subtle target movement (2, 7). Some reports have found that skin burns and pain in the treated regions were common and that severe complications, such as gastrointestinal perforation and SMA infarction, occurred after treatment with this type of HIFU machine (8, 9). However, such side effects should be avoided in patients with unresectable pancreatic cancers for the following reasons: they can destroy the quality of the remaining life, they can postpone chemotherapy, which is vital in these patients, and they can impair the patient's performance status. Indeed, a good performance status is known to be significantly associated with the median OS of pancreatic cancer patients (4).
Researchers have recently begun to focus more on pulsed HIFU therapy with low energy because animal and human research has indicated its potential to enhance the chemotherapeutic effect (10-13). In addition, pulsed HIFU with low energy employs much lower acoustic energy intensities (< 3 kW/cm2) than continuous HIFU, and exposure to these levels does not require hospitalization or general anesthesia and it has a low complication rate (13, 14). Accordingly, pulsed HIFU with a low energy appears to be a more reasonable, safer option for patients with unresectable pancreatic cancer because it allows patients to maintain staying on routine chemotherapy and have a normal life, and it offers the opportunity of achieving some benefit by enhancing the effect of chemotherapy.
Twelve patients were treated with pulsed HIFU with low energy from October 2008 to May 2010. In particular, three patients underwent concurrent chemotherapy and pulsed HIFU therapy (CCHT) with low energy. This study evaluated the potential clinical value of CCHT and the safety of pulsed HIFU for the treatment of unresectable pancreatic cancer, and here we summarize our experiences before we commence conducting a large prospective study.
Institutional Review Board approval was obtained for this retrospective study, and informed consent was waived due to the retrospective nature of this study.
Twelve patients were treated with HIFU from October 2008 to May 2010. Table 1 summarizes the patients' data. The 12 patients were determined to be eligible for HIFU treatment by using the following criteria: they had a diagnosis of pancreatic cancer as confirmed by the pathologic findings or by the clinical and imaging findings typical of pancreatic cancer, the tumor was unresectable based on the guidelines of the 6th edition of the American Joint Committee on Cancer (AJCC), the lesion was mainly located in the pancreatic head or body and they had a Karnofsky performance status scale rating of at least 70%.
The following inclusion criteria were used to examine the effect of CCHT: 1) the patients underwent at least three sessions of concurrent treatments, which were defined as HIFU within 24 hours of a gemcitabine injection, 2) initiation of the first CCHT was done within three months of the diagnosis to meet the requirements that concurrent treatment should be the main treatment and 3) there was no history of radiation or cyberknife treatment before and after CCHT. Three of the 12 patients satisfied these criteria and they were categorized into the "CCHT group." The other nine patients were categorized into the "non-CCHT group."
A FEP-BY™ HIFU unit (Yuande Biomedical Engineering Limited Corporation, Beijing, China) was used throughout this study (14). The HIFU treatment was performed using an upper HIFU transducer enveloped in a degassed water bladder with the patient lying supine on the treatment table. The targeted pancreatic tumor was identified using a B-mode ultrasound imaging transducer (GE Logiq 5, Seongnam, Republic of Korea) before the HIFU treatment. The HIFU beam was insonated into the body and moved automatically from spot to spot in an overlapping manner to treat a volume of tissue. Local or general anesthesia was not needed in any of the patients. The patients were fasted for nine hours before the HIFU treatment. The treatment parameters were input target energy: 500-1000 J/spot, input acoustic intensity (spatial average-temporal average intensity, Isata): 1-2 kW/cm2, pulses/spot: 50-70, transit time of a unit pulse (t1): 150 ms and intermission time between pulses (t2): 150 ms, intermission time between spots: 5 sec (duty cycle: 50%, pulse repetition frequency: 3.3 Hz, sonication duration: 15-21 sec). The acoustic power was adjusted with respect to the tumor depth and the subcutaneous tissue thickness, as measured by ultrasound imaging, to achieve the required in situ energy dose (15). To avoid skin burns, a cold ultrasound coupling gel was applied frequently between the water bladder and skin during the procedure.
Gemcitabine (2,2-difluorodeoxycytidine, Gemzar; Eli Lilly & Co., New York, NY) 1000 mg/m2 was administered as a 30 minute intravenous infusion weekly for three weeks followed by a one week rest period. Chemotherapy was omitted according to the physician's decision if a significant hematologic or nonhematologic toxicity had not recovered on the day of therapy. Within 24 hours of infusing gemcitabine, the HIFU treatment was conducted using the protocol described above. HIFU was generally cancelled when chemotherapy was cancelled due to an adverse reaction. However, the HIFU treatment alone was occasionally performed in cases with a long absence of treatment. Gemcitabine administration was continued until disease progression occurred.
Regarding the three patients in the CCHT group, the following were analyzed: the number of CCHT sessions, the number of HIFU alone treatments, the mean target energy per spot (J/spot), the mean input acoustic intensity (W/cm2), the mean treatment time from the first to last sonication, the serial changes in the level of the tumor marker CA 19-9, the serial changes of the tumor size by CT, the PET-CT findings if available, the OS from the time of diagnosis, the time to tumor progression (TTP), the presence of complications (redness, skin burn, treatment-related pain, pancreatitis, gastrointestinal injury and others) and the current performance status as determined by the Karnofsky scoring system.
The OS was calculated from the date of diagnosis to the date of death from any cause or the last documented follow-up. The TTP was calculated from the start of treatment to the date of the first documented progression or the last follow-up.
Regarding the nine patients in the non-CCHT group, we determined the main reason why CCHT was not performed more than twice. The OS from the time of diagnosis, the TTP and the presence or absence of complications during the HIFU treatment were also analyzed.
Tables 1 and 2 summarize the survival data, the treatment protocol and the complications of the CCHT group. Figures 1-3 show the serial changes in the CA 19-9 levels and the CT-determined tumor sizes.
Patient 1 (Fig. 1) (Tables 1, 2) had a 2.7 cm soft tissue lesion encasing the superior mesenteric artery (SMA) with an indistinct low attenuation area in the uncinate process of the pancreas, as seen on CT (Fig. 1A). The laparoscopic biopsy revealed atypical ductal cells and this suggested pancreatic carcinoma. PET-CT showed increased activity around the SMA. After gemcitabine was first administered in January 2009, CCHT was performed since January 2009 according to the gemcitabine infusion schedule. At the end of the first six sessions of CCHT, the CA 19-9 level had decreased from 50.8 U/mL to 21 U/mL. After six sessions of CCHT, three consecutive weekly CCHTs were performed with a 1-3 month intermission. The tumor size decreased and it remained stable in size for approximately one year since the first CCHT (Fig. 1B). However, subsequently, the CA 19-9 level began to be reelevated to more than 100 U/mL and the follow-up PET-CT revealed a small region of hot activity at the left paraaortic area that had not been treated with CCHT (Fig. 1D). Afterwards, the paraaortic lesion was treated with three consecutive sessions of CCHT. The follow-up CT (March 2010) showed that the attenuation of the left paraaortic area was reduced compared to the previous CT. On March 18, 2010, a new chemotherapeutic regimen (TS-1 and CDDP) was attempted due to a continuous increase in the CA 19-9 level. However, it was cancelled after the first treatment session due to severe adverse effects. Four consecutive sessions of CCHT with gemcitabine were performed from April 7, 2010 to April 28, 2010. After this treatment, the CA 19-9 level was decreased temporally (1341 U/mL → 1105 U/mL). Currently, the patient is refusing further chemotherapy. In September 2010, at the time of preparing this manuscript, he was still alive in a good physical condition (Karnofsky performance status scale: 80-90%), and there are no sign of distant metastases.
Patient 2 (Fig. 2) (Tables 1, 2) was diagnosed with a 3.3 cm unresectable pancreatic head cancer invading the peripancreatic tissue and the distal common bile duct with enlarged mesenteric lymph nodes according to the CT taken in July 2008. Three days later, drainage of the common bile duct was achieved via endoscopic retrograde cholangiopancreatography (ERCP) through the insertion of an uncovered metallic stent. At that time, the tumor was considered to be surgically resectable. However, the follow-up CT taken in September 2008 revealed an interval increase in the size of the main pancreatic mass (3.3 cm → 4.3 cm) and the mesenteric lymph nodes, and the new development of a small liver metastasis in segment 5 of the liver (Fig. 2A). Therefore, surgery was cancelled and a gemcitabine-capecitabine (Xeloda; F. Hoffmann-La Roche AG, Basel, Switzerland) combination was administered. The capecitabine was administered orally at a dose of 830 mg/m2 twice a day with gemcitabine being given once a week for the first three weeks. However, the capecitabine was discontinued due to hand-foot syndrome. CCHT was performed from November 2008. After the first three CCHT sessions, the CA 19-9 level decreased to 40.3 U/mL and the tumor size according to CT decreased to 2.8 cm in diameter (Fig. 2B). The liver nodule observed in segment 5 had completely disappeared according to the CT taken in May 2009. However, the CA 19-9 level was elevated to more than 100 U/mL in June 2009 and the CT in August 2009 revealed a slight interval increase in tumor size (2.5 cm → 2.9 cm in diameter). Surgical resection was considered given the lack of vital vessel involvement by the pancreatic tumor and his excellent physical condition (Karnofsky performance status scale: 100%). The liver lesion in segment 5 was not observed before surgery. After some delay of surgery due to cholangitis and a myocardial ischemic attack, the surgical resection (pylorus preserving pancreaticoduodenectomy) was performed in November 2009 (Fig. 2C). The pathological diagnosis was poorly differentiated adenocarcinoma, and the surgical staging was T3N1. No peritoneal seeding was identified. However, on a postoperative follow-up CT taken in November 2009, a single 1 cm nodule reappeared in segment 5 of the liver and it began to increase slowly in size, confirming that it was a liver metastasis (Fig. 2D). In addition, the portocaval lymph node had also increased slowly in size. A new chemotherapeutic regimen (TS-1 and CDDP) was started from April 2010. Thus far, the patient's Karnofsky performance statue scale is 90-100%, and no signs of another distant metastasis have been observed.
Patient 3 (Fig. 3) (Tables 1, 2) was diagnosed with a 4.6 cm infiltrative unresectable cancer of the pancreatic head with invasion of the distal common bile duct and encasement of both the celiac axis and the main portal vein in August 2008 (Fig. 3A). From the time of the diagnosis, there was a small quantity of ascites, which might represent peritoneal seeding (Fig. 3B). Therefore, the initial TNM staging was T4N1Mx (stage III or IV). In August 2008, ERCP was performed with the insertion of an uncovered metallic stent for drainage of the common bile duct. From August 2008, six sessions of a weekly gemcitabine infusion (2 cycles) were performed with capecitabine, which was administered orally at a dose of 830 mg/m2 twice daily for three weeks followed by a one week rest period. However, the CA 19-9 level increased (140 U/mL → 264 U/mL) and no definite change in tumor size occurred according to CT. CCHT was started in October 2008. The tumor was partially treated during every CCHT session because the upper half of the tumor around the celiac axis was always hidden by gastric gas on ultrasound. After the first three consecutive sessions of CCHT, the CA 19-9 level decreased (264 U/mL → 177 U/mL) as did the tumor size from 4.6 cm to 3.7 cm in diameter (Fig. 3B). The ascites also disappeared (Fig. 3C). In May 2009, when approximately two months had passed after his last CCHT, the CA 19-9 level was found to have increased abruptly to 1810 U/mL even though his physical performance status was excellent (Karnofsky score, 90%) and the CT did not reveal any significant interval change in tumor size at that time. In June 2009, his chemotherapeutic regimen was changed to FOLFOX (folinic acid [FOL] fluorouracil [F] and Oxalipatin [OX]), which unfortunately caused severe leucopenia and subsequent biliary sepsis (a known severe adverse reaction of the drug). Sadly, in July 2009, he succumbed to the biliary sepsis.
Of the 32 CCHTs and six HIFU alone treatments administered in the CCHT group, a superficial grade 2 skin burn occurred in patient 3, and a grade 1 skin burn was noted in patients 1, 2 and 3. The grade 2 skin burn measured approximately 4 × 5 cm in size and it required silver sulfadiazine ointment and a sterile gauze bandage for two weeks. It resolved completely within three weeks. The two grade 1 skin burns recovered without treatment within one week. No major complications such as gastrointestinal perforation or pancreatitis were encountered in the CCHT group. Mild abdominal pain was reported during treatment (during 2 sessions in patient 1, during 6 sessions in patient 2 and during 2 sessions in patient 3). Moderate abdominal pain was noted during one session in patient 1, during two sessions in patient 2 and during one session in patient 3. However, the pain was controlled with Fentanyl, and it disappeared immediately after completing the HIFU treatment.
The patients in this group did not undergo CCHT more than twice for the following reasons: intolerance of pain during treatment (n = 4), a poor physical condition due to disease progression (n = 3), palliative use of HIFU for pain relief (n = 1) and pancreatitis (n = 1). The patient who developed pancreatitis after the HIFU treatment had a small cystic portion close to the pancreatic cancer before treatment, which was probably a pseudocyst. This cystic lesion was included in the treatment field. Two weeks after the 3rd HIFU treatment, a large pseudocyst surrounded by inflammatory changes occurred in the mesentery anterior to the pancreas. This complication might have been caused by the delayed perforation of the cyst near the pancreatic tumor due to damage of the cystic wall by HIFU. All four patients in whom the HIFU treatment had been terminated due to severe pain during treatment were treated with more than 900 J/spot. The use of a high HIFU energy (> 900 J/spot) was favored for approximately two months after initiating HIFU treatment at our center, yet it frequently caused severe abdominal pain. Accordingly, the energy delivered was lowered to < 800 J/spot, and patients have since felt more comfortable during treatment. The patient who underwent HIFU for pain palliation did experience improvement of their pain (numeric pain scale 7 → 3).
The median OS and TTP of this group were 10.3 months (95% confidence interval [CI]: 1.4-19.3) and 4.4 months (95% CI: 0.0-9.0), respectively. A grade 1 skin burn occurred in only one of the nine patients, and this resolved within one week without any special treatment. Subcutaneous sclerosis caused by thermal injury to the subcutaneous fat of the anterior abdominal wall occurred in two patients, but this occurred after they had been administered 1000 J/spot. The sclerosis took four weeks to resolve without treatment in one patient. However, in the other, it was not completely resolved four months after completing the HIFU treatment.
The currently available treatment modalities for the management of patients with unresectable pancreatic cancer include chemotherapy, radiation therapy, preoperative neoadjuvant combined chemotherapy and radiation therapy (CCRT), postoperative adjuvant CCRT, etc. In the recent years, many institutions have been using CCRT for resectable or unresectable locally invasive pancreatic cancers because it has been reported to improve the local recurrence and survival rates (16, 17). However, CCRT cannot be persistently used because it is restricted by the total radiation dose. Therefore, after CCRT is finished, chemotherapy is only available treatment option in the current treatment scheme. On the other hand, CCHT can be persistently repeated because its use is not limited by the total dose. Therefore, if CCHT is clinically proven to be an effective modality for the treatment of pancreatic cancer by a well-designed study, then it can be used anytime in conjunction with the current treatment schemes to more improve the survival rates.
In the present study, two of the three patients in the CCHT group achieved survival times of 26.0 months and 21.6 months with an excellent physical condition and no major complications. The other patient also maintained a good physical condition for 11 months from the time of diagnosis without any significant increase in tumor size or de novo distant metastasis.
A recent clinical study was undertaken to examine concurrent gemcitabine and HIFU therapy in patients with locally advanced pancreatic cancer (13). In that previous study, the median OS was 12.6 months (95% CI: 10.2-15.0 months), which was an excellent result compared to the previous studies on chemotherapy alone (3, 4, 6), combined chemotherapy and radiotherapy (5) or HIFU therapy alone (2). That previous study excluded the cases of unresectable pancreatic cancer with metastatic disease. In our study, two patients in the CCHT group survived for longer than the upper limit of the 95% CI of the previous CCHT study, even though one of the two had liver metastasis. Furthermore, these two patients were still alive at the time of preparing this manuscript and they were in a near normal physical condition. The major difference between the CCHT protocols of the present and previous CCHT studies was the time interval between gemcitabine administration and the HIFU treatment. In the previous study, the patients received gemcitabine on days 1, 8 and 15 and HIFU therapy on days 1, 3 and 5. In other words, concurrent use of gemcitabine and HIFU was performed in the same day once per month. In contrast, the patients in our study received HIFU within 24 hours of gemcitabine administration in every CCHT.
According to our TTP, the CA 19-9 level and the CT findings, the period of growth inhibition appeared to extend beyond eight months from the time of initiating administering chemotherapeutic agents in all three patients, which, in our opinion, contributed to their excellent survival times. In several animal studies, CCHT induced apoptosis and it inhibited tumor growth more than chemotherapy or HIFU alone (10-12, 18). The mechanistic rationale for the inhibition of tumor growth by CCHT is based on the thermal and non-thermal effects of pulsed HIFU. Thermally, the pulsed HIFU induces an increase in blood flow to the target tissues by causing local hyperthermia, which increases drug delivery, and it also mechanically induces structural and molecular changes at the cellular and molecular levels due to acoustic cavitation, radiation force, shear stress and acoustic streaming/microstreaming, which all might enhance drug extravasation and sensitize the cancer cells to chemotherapeutic agents (1, 11).
The median OS and TTP of our non-CCHT group are similar to the results of the previous studies, with considering that the median OSs and median TTPs of gemcitabine and gemcitabine-capecitabine chemotherapies are 6-10 months and 3.8-5.4 months, respectively, for patients with advanced pancreatic cancer (3-6).
In terms of safety, no major complications, other than one event of pancreatitis, was encountered in either study group. However, mild pain was not uncommon during treatment, but it was well controlled with analgesics. When energy of 900-1000 J/spot was initially administered at our center, four patients presented with severe abdominal pain during the HIFU treatment and another two patients experienced a subcutaneous burn. However, no severe pain or subcutaneous burn has been encountered during treatment since this dose was reduced to 800 J/spot or less. Given that all three patients in the CCHT group received a mean target energy/spot of less than 850 J, a target energy of less than 800 J/spot might well be advisable.
HIFU carries a risk of biliary perforation or biliary duct damage by thermal injury when cancers are located in the head of the pancreas. To reduce the risk, a previous study recommended placing a biliary stent before HIFU in patients with cancer in the pancreatic head (2). In this current study, two patients with invasion of the distal common bile duct in the CCHT group underwent metallic biliary stent insertion due to obstructive jaundice before the initiation of CCHT. The biliary stents were always included in the treatment territory in all the HIFU sessions, and there was no evidence of a biliary obstruction or leakage in both patients. This suggests that metallic stents can prevent biliary complications. In addition, stents have the potential to augment the ablation of tumors around the stents by reflecting the HIFU beam, yet further study will be needed to validate this issue. Until the safety is validated, HIFU should be performed very carefully in patients with pancreatic head cancers, even though a metallic stent is inserted, because HIFU is potentially harmful to the bile ducts.
The present study had several limitations. First, the number of cases was small and the study was limited by its retrospective design. Applying an intention-to-treat principle revealed a technical success rate for CCHT of 25% (3 of 12 patients). However, this study highlighted the potential of CCHT using a practical treatment protocol prior to conducting any well-controlled prospective study. Second, a selection bias could not be avoided because the CCHT group consisted of patients with an excellent Karnofsky performance status at the time of the initial treatment, and a good performance status has a significant effect on the survival of patients with pancreatic cancer (4). Nevertheless, the survivals achieved by the three patients in the CCHT group were remarkable. These findings appear to justify conducting a well-designed prospective study to prove the value of CCHT in a more rigorous manner. Third, this study was also limited by its empirical nature in terms of determining the timing and CCHT doses, even though the acoustic intensity used in the study was based on that used in a previous study (15). Therefore, its validity should be confirmed in a further study.
In conclusion, CCHT has excellent potential to become an effective and safe modality for treating unresectable pancreatic cancer.
Figures and Tables
Fig. 1
60-year-old man with unresectable pancreatic cancer arising from uncinate process and encasing superior mesenteric artery.
A. Contrast-enhanced CT image (January 8, 2009) taken before start of concurrent chemotherapy and high intensity focused ultrasound therapy shows infiltrative soft tissue tumor (arrowheads) arising from uncinate process of pancreas with upward and downward encasement of superior mesenteric artery (arrows).
B. Contrast-enhanced CT image (February 20, 2009) taken after three consecutive concurrent chemotherapy and high intensity focused ultrasound therapy sessions shows slight interval decrease in tumor size and tumor soft tissue attenuation (arrowheads) around superior mesenteric artery (arrows).
C. Contrast-enhanced CT image (July 14, 2009) taken after additional three sessions of concurrent chemotherapy and high intensity focused ultrasound therapy shows another interval decrease in tumor size and amount of soft tumor tissue (arrowheads) encasing superior mesenteric artery (arrows).
D. PET-CT image (December 29, 2009) shows hot uptake (arrows) in left paraaortic area at level of superior mesenteric artery take-off. Left paraaortic area had not previously been treated with high intensity focused ultrasound because no mass was observed in this area on prior CT images. Note that treated soft tissue (arrowheads) around superior mesenteric artery does not show any significant uptake.
E. First plot demonstrates change in CA 19-9 concentration over time, showing that CA 19-9 levels were stable for approximately one year. Second plot shows change in tumor size (determined by CT) over time. After regression of tumor size, there was very slow change in size of main mass with time. Third plot shows date and number of chemotherapy sessions prior to enrollment and dates of concurrent chemotherapy and high intensity focused ultrasound (HIFU) therapy (CCHT) or high intensity focused ultrasound alone treatment.
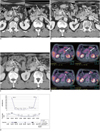
Fig. 2
53-year-old man with stage IV pancreatic body cancer and liver metastasis.
A. Contrast-enhanced CT (September 19, 2008) taken before start of concurrent chemotherapy and high intensity focused ultrasound therapy shows irregular soft tumor tissue (short white arrows) at pancreatic head with invasion to distal common bile duct. Multiple necrotic nodes (long white arrows) are shown in mesentery. Common bile duct contains metallic stent. Small hypodense lesion (black arrow) is present in segment 5, indicating metastasis.
B. Contrast-enhanced CT (May 15, 2009) taken after 4 sessions of concurrent chemotherapy and high intensity focused ultrasound therapy and 3 sessions of high intensity focused ultrasound therapy alone shows remarkable interval decrease of tumor size (arrows). Necrotic nodes present in mesentery were also reduced in size, and liver nodule disappeared (not shown).
C. Surgical specimen of pancreas after pylorus preserving pancreaticoduodenectomy (November 11, 2009) shows irregular whitish tumor (arrows) in pancreatic head. Pathological diagnosis was poorly differentiated adenocarcinoma.
D. Contrast-enhanced CT (January 9, 2010) taken two months after surgery shows reappearance of liver nodule (short arrows) in segment 5, suggesting relapse of liver metastasis. Slightly enlarged portocaval node was also observed (long arrow).
E. First plot shows change in CA 19-9 concentration with time. CA 19-9 level was increased again approximately 10 months after initial treatment. Second plot shows change of tumor size (determined by CT) with time. After initial regression, size of tumor remained relatively stable until surgery. Third plot shows dates and number of chemotherapy sessions prior to enrollment and dates of concurrent chemotherapy and high intensity focused ultrasound (HIFU) therapy (CCHT) or high intensity focused ultrasound alone treatment.
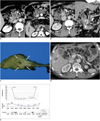
Fig. 3
61-year-old man with unresectable pancreatic head cancer with invasion to celiac axis and main portal vein.
A. Contrast-enhanced CT image (August 7, 2008) taken before start of concurrent chemotherapy and high intensity focused ultrasound therapy shows irregular bulky mass (white arrows) arising from pancreatic head with invasion to main portal vein and distal common bile duct (black arrow). Involvement of common hepatic artery (arrowheads) is shown on right of figure.
B. Contrast-enhanced CT (November 4, 2008) taken after one session of concurrent chemotherapy and high intensity focused ultrasound therapy shows partially interval decrease of attenuation of lower half of tumor (white arrows), which high intensity focused ultrasound could reach. Ascites is also shown (black arrow). Tumor infiltrations (arrowheads) around common hepatic artery and splenic artery, which high intensity focused ultrasound could not reach due to overlying gastric gas, are well shown in right of figure, and they show slight interval increase in extent.
C. Contrast-enhanced CT image (January 28, 2009) taken after additional 5 sessions of concurrent chemotherapy and high intensity focused ultrasound therapy shows stable tumor size (arrows) compared to prior CT dated November 4, 2008. Tumor infiltration (arrowheads) around common hepatic artery and splenic artery is stable in extent. Ascites had disappeared (not shown).
D. First plot shows change in CA 19-9 concentration with time. CA 19-9 level was stable for approximately eight months. Second plot shows change in tumor size (determined by CT) with time. After initial tumor regression, it remained relatively stable until patient succumbed to drug induced complications. Third plot shows dates and number of chemotherapy sessions prior to enrollment and dates of concurrent chemotherapy and high intensity focused ultrasound (HIFU) therapy (CCHT) or high intensity focused ultrasound alone treatment.
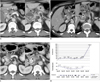
Table 1
Summary of Patients Treated with High Intensity Focused Ultrasound

Note.-CCHT = concurrent chemotherapy and pulsed high intensity focused ultrasound therapy, CI = confidence interval, Current status = patient's status at time of writing, HIFU = high intensity focused ultrasound, M/D = moderately differentiated, No. = number, P/D = poorly differentiated, TNM = tumor, node, metastasis, TTP = time to tumor progression
References
1. Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol. 2008. 9:291–302.
2. Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005. 236:1034–1040.
3. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009. 27:5513–5518.
4. Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007. 25:2212–2217.
5. Park JK, Ryu JK, Lee JK, Yoon WJ, Lee SH, Kim YT, et al. Gemcitabine chemotherapy versus 5-fluorouracil-based concurrent chemoradiotherapy in locally advanced unresectable pancreatic cancer. Pancreas. 2006. 33:397–402.
6. Park BB, Park JO, Lee HR, Lee J, Choi DW, Choi SH, et al. A phase II trial of gemcitabine plus capecitabine for patients with advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2007. 60:489–494.
7. Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol. 2001. 27:1099–1106.
8. Li JJ, Gu MF, Luo GY, Liu LZ, Zhang R, Xu GL. Complications of high intensity focused ultrasound for patients with hepatocellular carcinoma. Technol Cancer Res Treat. 2009. 8:217–224.
9. Li JJ, Xu GL, Gu MF, Luo GY, Rong Z, Wu PH, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol. 2007. 13:2747–2751.
10. Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007. 13:2722–2727.
11. Poff JA, Allen CT, Traughber B, Colunga A, Xie J, Chen Z, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008. 248:485–491.
12. Wang RS, Liu LX, Gu YH, Lin QF, Guo RH, Shu YQ. The effect of endostatin and gemcitabine combined with HIFU on the animal xenograft model of human pancreatic cancer. Biomed Pharmacother. 2010. 64:309–312.
13. Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010. 21:447–452.
14. Xiong LL, Hwang JH, Huang XB, Yao SS, He CJ, Ge XH, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009. 10:123–129.
15. Hwang JH, Wang YN, Warren C, Upton MP, Starr F, Zhou Y, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009. 35:967–975.
16. White RR, Hurwitz HI, Morse MA, Lee C, Anscher MS, Paulson EK, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001. 8:758–765.
17. Wolff RA. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas: great logic, grim reality. Ann Surg Oncol. 2001. 8:747–748.
18. Paparel P, Chapelon JY, Bissery A, Chesnais S, Curiel L, Gelet A. Influence of the docetaxel administration period (neoadjuvant or concomitant) in relation to HIFU treatment on the growth of Dunning tumors: results of a preliminary study. Prostate Cancer Prostatic Dis. 2008. 11:181–186.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download