Abstract
Acinar cystic transformation of the pancreas is also known as acinar cell cystadenoma (ACC), and this is an extremely rare benign lesion that was first described in April 2002. We report here on a case of a previously asymptomatic patient with pancreatic ACC and this was diagnosed by computed tomography (CT) and magnetic resonance imaging (MRI). To the best of our knowledge, there is no previous report concerning the CT or MRI features of ACC in the medical literature. We present here the CT, MRI and pathological findings of pancreatic ACC.
Acinar cell tumors of the pancreas are uncommon and they include acinar cell carcinomas, acinar cell cystadenocarcinomas and acinar cell cystadenomas (ACCs) (1-5). These tumors are composed of typical round or cylindrical cells with eosinophilic periodic acid stain (PAS)-positive cytoplasmic granules. On immunohistochemical analysis, they express pancreatic enzymes, and electron microscopy demonstrates that they contain zymogen granules (3). Acinar cell cystadenocarcinoma and ACC, which are both lined by acinar cells, are extremely rare tumors; moreover, ACC was an unknown lesion until year 2002.
We report here on a previously asymptomatic patient with pancreatic ACC and who was diagnosed by computed tomography (CT) and magnetic resonance imaging (MRI) and the diagnosis was later confirmed both surgically and pathologically. We present the CT, MRI and histopathological findings of pancreatic ACC. To the best of our knowledge, there has been no previous report concerning the CT and/or MRI features of ACC in the medical literature.
A 52-year-old male with a one-year history of renal cell carcinoma was admitted for routine follow-up. The general physical examination and laboratory tests were normal. We observed hypodense-hypovascular cystic lesions with thin septi in the uncinate process and body of the pancreas, and the pancreas measured 5 cm in diameter on the abdominal CT scan (Fig. 1A). Contrast-material enhanced MRI revealed numerous diffusely distributed cysts with thin septi, and these cysts ranged in size from a few millimeters to 14 mm in diameter in the body and uncinate process of the pancreas. Contrast-material enhancement in the pancreatic lesions was not observed on the postcontrast (arterial and portal venous phase) T1 weighted (W) images (Fig. 1B-D). The main pancreatic and extrahepatic bile ducts were normal on the T2W sequences. Our preoperative diagnosis was "cystic neoplasm of the pancreas", and pancreaticoduodenectomy was performed by general surgeons.
Macro- and microscopic examination revealed numerous cysts ranging from 2 to 12 mm in diameter with a thin translucent wall in the body and head of the pancreas. The tumor was 5 cm in diameter. The internal surface of the cysts was pink, smooth and glistening and the surface of the cysts had no solid areas or papillary projections. There was no discrete tumoral lesion, necrosis, calcification or hemorrhage observed on further examination of the pancreas (Fig. 1F). The duodenum was normal in appearance.
Microscopic examination revealed numerous small and large cysts diffusely distributed throughout the pancreatic tissue. The cysts seemed to develop from the surrounding acinar tissue and they were lined by cells with the typical features of normal acinar cells. There were disseminated patchy acinar structures within some small and large cyst walls (Fig. 1E, F). The small cysts were lined by a single layer of bland cuboidal or columnar acinar cells with tendency for crowding. Their nuclei were basally located and regular, and they contained small nucleoli. The cells' cytoplasm was typical for acinar cells with deeply eosinophilic granules in the apical part. The large cysts were lined by a single layer of flattened, low, cuboidal acinar epithelium and these cells had basally oriented nuclei. The cysts were generally connected to small clusters of acinar cells forming acini, which opened into the cyst lumen. Eosinophilic secretion was demonstrated in some cystic structures.
A foveolar metaplasia-like area was seen in a minute focus. Immunohistochemically, the acinar cells of the cysts (small or large) and areas of cystic transformation were positive for cytokeratin 7, while the normal acinar cells were negative for cytokeratin 7. Both the normal acinar cells and the epithelial lining cells were positive for cytokeratin and epithelial membrane antigen, but they were negative for S-100 and progesterone. Histochemically, the eosinophilic apical cytoplasm of the cuboidal acinar cells and the acinar cells of the cysts were PAS-positive. Both the normal acinar cells and the acinar cells associated with the cyst epithelium were negative for Alcian blue pH 2.5. The acinar cells of the cysts (small or large), the acinar structures with cystic transformation and the normal acinar structures were negative for synaptophysin and chromogranin, while the normal islet cells were positive for synaptophysin and chromogranin. The acinar cells associated with the cyst epithelium and the normal acinar cells were negative for p53, and their Ki67 index was less than 1%. As a result of these findings, the histopathologic diagnosis was ACC of the pancreas. The patient is still alive at the postoperative sixth month with no evidence of pancreatic cystic lesion.
Until recently, the conventional thought was that all acinar neoplasms (solid or cystic) in the pancreas are malignant (4). Acinar cystic transformation (ACT) of the pancreas is also known as ACC, and it is an extremely rare entity. This benign lesion was first described in April 2002 by Albores-Saavedra as "acinar cell cystadenoma" (6). There have been only a few case reports on this tumor in the pathology literature up to now (6-9). ACC has a tendency to be seen in young adults and it is accepted as a benign process (4). All of the reported patients in the literature were symptomatic and/or they had additional diseases; however, our patient was completely asymptomatic (5-9) (Table 1).
The etiology of ACT is still unknown. In spite of the additional information provided by electron microscopy and immunohistochemistry, the definitive histogenesis of this lesion remains controversial. Various theories have been put forward in regard its neoplastic or non-neoplastic origin (7). Chatelain et al. (8) preferred to call the lesion a "cystic tumor" since it is not related to the normal acini and they suggested that it is the cystic counterpart of acinar cell adenoma. Likewise, Albores-Saavedra mentioned that the cysts might be metaplastic and of a ductal origin since they are lined by ductal-like cells (6). Nevertheless, he suggested certain facts such as the tumor has a large multi-loculated structure and the lack of ductal obstruction, chronic inflammation and fibrosis in the adjacent pancreatic tissue eliminates the probability of this being a non-neoplastic lesion (6).
In our case, there was no discrete solid lesion or well-defined fibrous pseudocapsule; numerous cysts were diffusely distributed throughout the pancreas tissue. The cysts seemed to develop from the surrounding acinar tissue and they were lined by cells with the typical features of normal acinar cells. Moreover, some cysts were connected to normal pancreatic acini. Therefore, we suggest the description of this condition as "acinar cystic transformation or a sponge pancreas". The same as happened in our case, most of the previously reported ACTs were misdiagnosed as cystic tumor by the preoperative radiologic examination. Hence, extensive surgery was carried out. Histopathologic examination after resection is necessary for making the final diagnosis. On the other hand, one should be aware that the histologic appearance might be confused with that of the cystic variant of acinar cell carcinomas, the serous or mucinous cystic neoplasms and other rare cystic lesions of the pancreas. Based on the macroscopic, histologic, histochemical and immunohistochemical findings, ACT can be easily distinguished from these lesions since the cysts lined by acinar cells without cellular atypia and mitoses are only observed in ACT of the pancreas.
Radiologically, making the differential diagnosis between a cystic neoplasm and pseudocyst is very important (10, 11). However, the radiographic features of cystic pancreatic neoplasms remain uncertain. The thick-walled pancreatic pseudocysts may resemble acinar cell tumors (11). Patients with pseudocysts generally have a history of acute pancreatitis, whereas those with cystic tumors most often lack such a history (12, 13). Evidence of inflammatory changes or calcifications in the pancreas is suggestive of a pancreatic pseudocyst (11). The presence of a contrast-material-enhanced central fibrosis that is visualized with the use of CT or MR images is a highly diagnostic feature that is found in about 30% of serous cystadenomas (13, 14).
Intraductal papillary mucinous tumors (IPMTs) may involve the main pancreatic duct exclusively, a side branch of the main duct or both (14). IPMT can be differentiated with MRI, and particularly with using the heavily T2 weighted or MR cholangiopancreaticography (MRCP) images (13). Lymphoepithelial cyst should also be considered in the differential diagnosis of cystic neoplasms of the pancreas (15). Ultrasound-guided aspiration of the cystic fluid may be helpful in cases with pancreatic cystic lesion; while the levels of amylase are increased in the cystic fluid of pseudocysts, the carcinoembryonic antigen levels are elevated in many mucinous cystic tumor cases and keratinous and amorphous debris can be seen in lymphoepithelial cysts (13, 15). The presence of a well-delineated enhancing capsule or internal punctate calcification in a pancreatic tumor should prompt including acinar cell cystadenocarcinoma in the differential diagnosis (16, 17).
In conclusion, the CT and MRI appearance of ACT is a well-delineated, homogeneous hypovascular cystic mass with thin septation, and these tumors are hypodense on CT images, hypointense on the pre- and postcontrast T1W images and hyperintense on the T2W images. Although ACT is a rare pancreatic tumor, recognition of these CT and MRI features may help radiologists to arrive at the correct diagnosis in some cases (16). Accurate identification and appropriate treatment of these lesions are quite important, and particularly in terms of preventing extensive surgery.
Figures and Tables
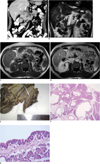 | Fig. 1Pancreas acinar cell cystadenoma in 52-year-old man.
A. Contrast-material enhanced, coronal reformatted multidetector CT image. Multiple hypodense cystic lesions are clearly seen in pancreas (arrows).
B-D. Coronal half-Fourier acquisition single-shot turbo spin-echo (HASTE) (B), axial precontrast (C) and postcontrast T1 weighted (D) MR images of pancreas. Nonenhancing cystic lesions are seen in pancreas (arrows).
E. Image of macroscopic specimen. Numerous cysts with thin translucent wall are seen in body and head of pancreas.
F, G. Numerous cystic spaces are diffusely distributed in pancreas tissue (F). Note small clusters of acinar cells forming acini that open into cyst lumens (G) (Hematoxylin & Eosin stain, original magnification, × 20 [F], 600 [G]).
|
Table 1
Clinicopathological Features of Reported Cases of Acinar Cystic Transformation of Pancreas
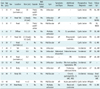
Note.- *= months, **= our case, AMI = acute myocardial infarction, AP = abdominal pain, AW = alive and well (this table was modified from reference 7 with permission), D = diabetes, DP = distal pancreatectomy, ID = internal drainage of the two cysts, IPA = intraductal papillary adenoma, LP = left pancreatectomy, NDG = nodular diabetic glomerulosclerosis, NEOCG = no evidence of cystic growth, NEOD = no evidence of disease, No. = number, PA = polyarthralgia, PD = pancreaticoduodenectomy, TP = total pancreatectomy, uncinate p. = uncinate process, yr = years
References
1. Hamilton SR, Aaltonen LA. WHO classification of tumours. Pathology and genetics of tumours of digestive system. 2000. Lyon, France: IARC Press.
2. Huang Y, Cao YF, Lin JL, Gao F, Li F. Acinar cell cystadenocarcinoma of the pancreas in a 4-year-old child. Pancreas. 2006. 33:311–312.
3. Solcia E, Capella C, Kloppel G. Tumors of the pancreas. Atlas of tumor pathology. 1997. Washington, DC: AFIP;103–114. 3rd Series, Fascicle 20.
4. Adsay NV. Cystic lesions of the pancreas. Mod Pathol. 2007. 20:S71–S93.
5. Cantrell BB, Cubilla AL, Erlandson RA, Fortner J, Fitzgerald PJ. Acinar cell cystadenocarcinoma of human pancreas. Cancer. 1981. 47:410–416.
6. Albores-Saavedra J. Acinar cystadenoma of the pancreas: a previously undescribed tumor. Ann Diagn Pathol. 2002. 6:113–115.
7. Zamboni G, Terris B, Scarpa A, Kosmahl M, Capelli P, Klimstra DS, et al. Acinar cell cystadenoma of the pancreas: a new entity? Am J Surg Pathol. 2002. 26:698–704.
8. Chatelain D, Paye F, Mourra N, Scoazec JY, Baudrimont M, Parc R, et al. Unilocular acinar cell cystadenoma of the pancreas an unusual acinar cell tumor. Am J Clin Pathol. 2002. 118:211–214.
9. Couvelard A, Terris B, Hammel P, Palazzo L, Belghiti J, Lévy P, et al. Acinar cystic transformation of the pancreas (or acinar cell cystadenoma), a rare and recently described entity. Ann Pathol. 2002. 22:397–400. [French].
10. Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, et al. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005. 184:511–519.
11. Brugge WR. Cystic neoplasms of the pancreas. Endoscopic oncology. 2006. Totowa, New Jersey: Humana Press Inc;289–294. Chapter 24.
12. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005. 25:1471–1484.
13. Acar M, Tatli S. Cystic tumors of the pancreas: a radiological perspective. Diagn Interv Radiol. 2010. [Epub ahead of print]. DOI: 10.4261/1305-3825.DIR.3254-09.1.
14. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004. 351:1218–1226.
15. Kazumori H, Sizuku T, Ueki T, Uchida Y, Yamamoto S. Lymphoepithelial cyst of the pancreas. J Gastroenterol. 1997. 32:700–703.
16. Chiou YY, Chiang JH, Hwang JI, Yen CH, Tsay SH, Chang CY. Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr. 2004. 28:180–186.
17. Chung WJ, Byun JH, Lee SS, Lee MG. Imaging findings in a case of mixed acinar-endocrine carcinoma of the pancreas. Korean J Radiol. 2010. 11:378–381.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download