Abstract
Follicular dendritic cell sarcoma is a rare malignant neoplasm and little is known about its radiological features. We present here four cases of follicular dendritic cell sarcomas and we provide the image characteristics of these tumors to help radiologists recognize this entity when making a diagnosis.
Follicular dendritic cell sarcoma is an extremely rare malignant neoplasm arising from follicular dendritic cells, and this was first described in 1986 by Monda et al. (1). About 60% of the cases occur in lymph nodes such as the cervical, axillary and mediastinal lymph nodes, while a wide variety of extranodal sites may also be affected, including the tonsil, spleen, liver, gastrointestinal tract, mediastinum and breast (2). Approximately one hundred cases of follicular dendritic cell sarcoma have currently been reported in the English medical literature. However, to the best of our knowledge, the majority of them have focused on the pathology and etiology, and little emphasis has been placed on the radiological appearance of this tumor, except for two articles that described the radiographic features of one mediastinal and two intraabdominal follicular dendritic cell sarcomas (3, 4). We present here the images of four cases: one lesion occurred in the mediastinum, another lesion occurred in the abdomen and two lesions occurred in the neck. Our objectives were to provide the image characteristics of this rare sarcoma and to help radiologists recognize it when making a diagnosis.
A 47-year-old man complained of chest malaise and pain that had persisted for about eight months. He had no fever, cough, expectoration or decompensation during the course of disease.
The unenhanced CT images of the chest demonstrated a well-defined mass of homogeneous attenuation in the posterior mediastinum, and the mass measured 7.5 × 4 cm in diameter on CT. An arborizing-pattern of coarse calcifications could be seen within the lesion (Fig. 1A). After the administration of intravascular contrast media, the mass showed intense homogeneous enhancement to a degree that was similar to the enhancement of the adjacent great vessels (Fig. 1B). The mass compressed the left atrium and the right pulmonary artery. The esophagus was posterolaterally displaced. A small hypodense region could also be seen in the tumor. Enlarged lymph nodes were found in the paratracheal and aortopulmonary regions.
Surgical excision of the tumor was attempted at an outside hospital under the impression of a neurogenic tumor, but this failed due to massive tumoral hemorrhage during the surgery and a biopsy could only be performed. The histopathological and immunohistochemical examinations revealed follicular dendritic cell sarcoma. The patient then underwent radiotherapy and he has been alive with the disease for 14 months.
A 28-year-old woman presented with an insidious onset of upper abdominal pain and this was accompanied by sour regurgitation and eructation, and this had all started two months ago. She had been treated under the diagnosis of having gastritis for four weeks at an outside hospital, but the symptoms had not improved.
An air-barium double-contrast upper gastrointestinal series disclosed wall stiffness in the lesser curvature of the stomach and broadening of the incisura, suggesting an extrinsic compression. The gastric mucosal surface appeared smooth and regular. No obvious niche sign or filling defect was detected (Fig. 2A). An unenhanced CT scan showed a large well-circumscribed mass of heterogeneous attenuation between the lesser curvature of the stomach and the left lobe of the liver, and the mass was about 11 × 7 × 10 cm in dimension, as measured on CT. The lesion was generally hypodense compared with the liver and there were even lower density regions scattered within the lesion (Fig. 2B). After intravenous contrast enhancement, the tumor was moderately enhanced and it was somewhat heterogeneously enhanced on the arterial phase. Several prominent feeding vessels were noted in the periphery of the tumor (Fig. 2C). The mass was heterogeneously hypodense compared with the hepatic parenchyma during the portal phase (Fig. 2D). No enlarged lymph nodes were found in the retroperitoneum.
A gastrointestinal stromal tumor was suspected and so radical subtotal gastrectomy was then performed. The excised mass measured 15 cm in diameter and it was found to have originated in the submucosa of the stomach. Central necrosis was observed in the tumor. The overlying gastric mucosa was intact. The histopathological and immunohistochemical findings (Fig. 2E, F) confirmed the diagnosis of follicular dendritic cell sarcoma.
Three months after the surgery, a follow-up MRI showed a 1.7-cm nodule in the right lobe of the liver, and the nodule had low signal intensity on the T1-weighted images (Fig. 2G) and hyperintensity on the T2-weighted images with a hypointense center (Fig. 2H). On the T1-weighted gadolinium-enhanced images, the nodule showed homogeneous enhancement during the arterial phase, making it appear isointense compared to the surrounding normal liver (Fig. 2I). The lesion appeared slightly hypointense compared to the liver during the portal venous phase (Fig. 2J). Wedge lobectomy of the liver with including the tumor was performed and this was followed by adjuvant chemotherapy. Pathological examination confirmed a metastatic follicular dendritic cell sarcoma. Metastases re-developed in the liver nine months thereafter. The MRI features of the new metastatic lesions were similar to those of the prior metastasis. The patient has been alive with the disease for the following two years.
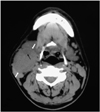
A 38-year-old man presented with a two-week history of a common cold and a painless right cervical mass. The axial unenhanced CT demonstrated a 4.5-cm right submandibular mass (Fig. 3) that was displacing the right submandibular gland anteromedially, the right sternocleidomastoid muscle posterolaterally and the right carotid artery and the right internal jugular vein anteromedially. The mass had a well-delineated margin and it generally showed homogeneous soft-tissue attenuation that was similar to the attenuation of the submandibular gland and slightly lower than that of the adjacent neck muscles. A small area of slightly lower attenuation was also seen in the mass. Subsequently, the mass was excised and it was diagnosed as follicular dendritic cell sarcoma of a cervical lymph node. The patient underwent adjuvant radiation therapy to the right side of the neck and he is alive with no evidence of disease recurrence for 16 months.
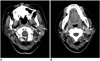
A 35-year-old woman first presented in 1998 with a left postauricular mass that was then excised at an outside hospital (the pathological diagnosis was unavailable). In 2005, a mass, which was presumably a local recurrence of the previous disease, developed in the similar left periauricular location and this was re-excised at another hospital, and the mass was pathologically confirmed as an ectopic meningioma. In late 2007, the patient visited our hospital for recurred masses in the left periauricular location. Fine needle aspiration of the periauricular masses was performed, and the results were suspicious for a malignant tumor.
The axial contrast-enhanced CT scan of the neck (Fig. 4) demonstrated multiple masses, which were assumed to be enlarged lymph nodes, in the left parotid gland region and in the retrocervical space, and the masses ranged from 1.5 to 3 cm in diameter. The nodes showed homogeneous moderate enhancement.
The patient underwent a left radical neck dissection. The histopathological examination confirmed the diagnosis of follicular dendritic cell sarcoma that originated from the cervical lymph nodes. The patient has remained disease-free for eight months after the surgery.
In the current study, the sizes of the follicular dendritic cell sarcomas of the four patients ranged from 1.5 to 15 cm. The mediastinal (7.5 cm) and intraabdominal lesions (15 cm) were much larger than the lesions located in the neck (4.5 cm and 1.5 cm). Consistent with our results, some previous studies (5-7) have also suggested that the intraabdominal tumors were bigger than those that occurred outside the abdominal cavity. According to the cases of follicular dendritic cell sarcoma reported in the literature (3-11), the average sizes of the 38 reported abdominal lesions and the 11 mediastinal lesions were 10.7 cm (range: 3.5 to 22 cm) and 6.7 cm (range: 3 to 10 cm), respectively, whereas 18 of the 19 lesions in the neck (and that originated from the cervical lymph nodes) were smaller than 6 cm. The tumor margins of our cases were generally well defined. Their CT attenuation was variable, and the tumors demonstrated relatively homogeneous attenuation in the lesions of the cervical lymph nodes, but they showed heterogeneous attenuation in the large abdominal lesion. Similar to our case of abdominal follicular dendritic sarcoma, areas of gross intratumoral necrosis or hemorrhage were noted in 32 of the 38 (84%) intraabdominal cases reported in the literature and Kang et al. (4) suggested a well-defined mass with internal necrosis was the CT findings of a follicular dendritic cell sarcoma of the abdomen. The presence of gross necrosis in the abdominal lesions may be related to the tumor size, as a large tumor is prone to cystic degeneration or necrosis.
Few studies have evaluated the vascularity of follicular dendritic cell sarcomas. Leipsic et al. (3) suggested indistinctly that a follicular dendritic cell sarcoma might be a relatively hypovascular lesion according to their surgery report, but this was not consistent with our findings. In our study, the first case in the mediastinum showed marked enhancement after the administration of intravascular contrast media and the tumor was shown to be hypervascular at surgery. Also, in the second case in the abdomen, prominent feeding vessels were noted in the lesion's periphery, although the degree of tumor enhancement after contrast administration was lower than that of the liver. These results may suggest that at least some follicular dendritic cell sarcomas are not hypovascular as was reported previously (3). More cases are necessary to clarify the vascularity of follicular dendritic cell sarcomas.
In our study, the CT findings of the first case that occurred in the mediastinum, including a round homogeneous mass, intense enhancement and arborizing calcifications, were quite similar to those of Castleman disease (12). "Castleman disease-like" intratumoral calcification was also noted in another previously reported mediastinal lesion (3). Moreover, a subset of follicular dendritic cell sarcomas has been reported to arise in or concomitantly with foci of Castleman disease (3). Therefore, the radiological distinction between follicular dendritic cell sarcoma and Castleman disease in the mediastinum could be quite difficult and this needs more accumulated cases and further investigation.
Some of the cases of follicular dendritic cell sarcomas reported in the literature and that involved the gastrointestinal tract were initially misdiagnosed as gastrointestinal stromal tumors (13, 14). Similarly, our second case was initially misdiagnosed as a gastrointestinal stromal tumor. Even if a follicular dendritic cell sarcoma is included in the differential list for a large stromal tumor-like abdominal mass seen on imaging, making a further specific diagnosis is not possible due to the generally nonspecific nature of the imaging findings.
The two cases of follicular dendritic cell sarcomas that involved the cervical lymph nodes in our series presented as well-delineated homogeneous masses. These features may help distinguishing follicular dendritic cell sarcomas from cervical metastatic carcinoma or schwannoma, as the latter tumors often show heterogeneous lesion attenuation due to central necrosis and infiltrating borders.
Follicular dendritic cells are antigen capture/presenting cells and they are located not only in the lymph nodes but also in extranodal sites. So it is possible that follicular dendritic cell sarcoma may occur in diverse sites throughout the body (5, 15). Follicular dendritic cell sarcoma is predominantly a disease of young adults. There is no gender predilection (6, 7). About 60% of patients present with painless cervical or axillary lymph node enlargement. Other symptoms include nonspecific epigastralgia, cough, weight loss and anemia (16). The behavior of this tumor is believed to be like that of a low-grade sarcoma and surgical excision is the primary treatment for most patients (6, 7). Local recurrence after surgical resection is not uncommon and the usual metastatic sites are the lung, liver and lymph nodes (5). The previous studies also showed that intraabdominal tumors are invariably aggressive and prone to distant metastasis (6, 7).
For the histopathological findings, a follicular dendritic cell sarcoma may be suspected if the tumor has distinct microscopical features such as a storiform arrangement of spindle shaped cells, indistinct cell borders and a background of lymphocytes scattered throughout the neoplastic cells (5). However, immunohistochemical staining, including CD21 and CD35, which are the most widely used follicular dendritic cell markers, are necessary to distinguish follicular dendritic cell sarcoma from other spindle cell neoplasms (5). S-100, CD117 and some muscular or vascular markers may also help in distinguishing it from other tumors such as malignant peripheral nerve sheath tumor and gastrointestinal stromal tumor. In our third case, the lesion may have initially been misdiagnosed as ectopic meningioma due to the similar histologic findings between follicular dendritic cell sarcoma and meningioma.
It is our impression that follicular dendritic cell sarcomas can have variable radiological features that are generally nonspeciflc. In our series, the tumors were well deflned and of variable sizes. The attenuation was homogenous in the small tumors, but areas of low attenuation due to internal necrosis were found in the large lesions. Hypervascularity and calciflcation were noted in one mediastinal lesion.
Figures and Tables
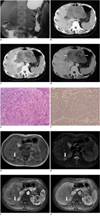
Fig. 1
Follicular dendritic cell sarcoma in mediastinum in 47-year-old man.
A. Unenhanced CT image of thorax reveals well-defined posterior mediastinal mass of homogeneous attenuation (white arrows) with arborizing-pattern of calcification (black arrow).
B. Contrast-enhanced CT image shows marked homogeneous enhancement of mass. Note compression of left atrium (black asterisk) and displacement of esophagus (white arrow).

Fig. 2
Follicular dendritic cell sarcoma in upper abdomen in 28-year-old woman.
A. Image of air-barium double-contrast study shows broadening of incisura due to extrinsic compression along lesser curvature of stomach (black arrows). Overlying mucosa appears to be intact. B. Unenhanced CT image of upper abdomen shows large heterogeneous mass (white arrows) located between stomach and left lobe of liver. C. Contrast-enhanced CT image during arterial phase shows heterogeneous moderate enhancement of tumor. Note feeding arteries in periphery of tumor (black arrows). D. Portal venous phase image shows heterogeneous moderate contrast enhancement of tumor (white arrows). E. Histopathological appearance reveals that tumor is composed of spindle cells that are arranged in storiform and whorled pattern and these spindle cells are admixed with lymphocytes (Hematoxylin & Eosin stain, × 100). F. Tumor shows positive immunohistochemical staining for CD21 (paraffin immunohistochemical stain, × 100). G. T1-weighted image shows hypointense metastatic nodule in right lobe of liver (white arrow). H. Nodule is hyperintense with hypointense center on T2-weighted image (white arrow). I. T1-weighted arterial-phase contrast-enhanced image shows isointensity of nodule (white arrow) due to homogeneous enhancement. J. T1-weighted portal-phase enhanced image shows heterogeneous, mild hypointensity of nodule (white arrow).
References
1. Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986. 122:562–572.
2. Weiss LM, Grogan TM, Muller-Hermelink H-K, Stein H, Pura T, Favara B, et al. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. Histiocytic and dendritic cell neoplasms. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC Press;286–288.
3. Leipsic JA, McAdams HP, Sporn TA. Follicular dendritic cell tumor of the mediastinum. AJR Am J Roentgenol. 2007. 188:W554–W556.
4. Kang TW, Lee SJ, Song HJ. Follicular dendritic cell sarcoma of the abdomen: the imaging findings. Korean J Radiol. 2010. 11:239–243.
5. Shia J, Chen W, Tang LH, Carlson DL, Qin J, Guillem JG, et al. Extranodal follicular dendritic cell sarcoma: clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. 2006. 449:148–158.
6. Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997. 79:294–313.
7. Perez-Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol. 1996. 20:944–955.
8. Shek TW, Liu CL, Peh WC, Fan ST, Ng IO. Intra-abdominal follicular dendritic cell tumour: a rare tumour in need of recognition. Histopathology. 1998. 33:465–470.
9. Díaz de Liaño A, Garde C, Artieda C, Yárnoz C, Flores L, Ortiz H. Intra-abdominal follicular dendritic cell sarcoma. Clin Transl Oncol. 2006. 8:837–838.
10. Jiang L, Admirand JH, Moran C, Ford RJ, Bueso-Ramos CE. Mediastinal follicular dendritic cell sarcoma involving bone marrow: a case report and review of the literature. Ann Diagn Pathol. 2006. 10:357–362.
11. Ceresoli GL, Zucchinelli P, Ponzoni M, Gregorc V, Bencardino K, Paties CT. Mediastinal follicular dendritic cell sarcoma. Haematologica. 2003. 88:ECR04.
12. Ko SF, Hsieh MJ, Ng SH, Lin JW, Wan YL, Lee TY, et al. Imaging spectrum of Castlemans' disease. AJR Am J Roentgenol. 2004. 182:769–775.
13. Chang KC, Jin YT, Chen FF, Su IJ. Follicular dendritic cell sarcoma of the colon mimicking stromal tumour. Histopathology. 2001. 38:25–29.
14. Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol. 2006. 36:249–253.
15. O'Malley DP. Diagnosis: follicular dendritic cell tumor mimicking GI stromal tumor. 2004. In: http://socforheme.org/case-nov-04.htm.
16. Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol. 2001. 14:354–360.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download