Abstract
The detection of thyroid nodules has become more common with the widespread use of ultrasonography (US). US is the mainstay for detecting and making the differential diagnosis of thyroid nodules as well as for providing guidance for a biopsy. The Task Force on Thyroid Nodules of the Korean Society of Thyroid Radiology has developed recommendations for the US diagnosis and US-based management of thyroid nodules. The review and recommendations in this report have been based on a comprehensive analysis of the current literature, the results of multicenter studies and from the consensus of experts.
Thyroid nodules are a common clinical problem and the incidence of thyroid nodules has increased with the recently increased use of thyroid ultrasonography (US). Several previous studies have demonstrated that thyroid nodules are found in 4-8% of the general population with the use of palpation, in 19-67% of patients with the use of US and in 50% of autopsy specimens (1-4). Malignancies have been found in 9-15% of the nodules that were evaluated with fine-needle aspiration (FNA) biopsy (1-5). The same as in other countries, the incidence of thyroid cancer is rapidly increasing in Korea and it is becoming the most common cancer in Korean women, followed by breast cancer, according to the recent report (6).
Thyroid nodules are especially more common in elderly patients, female patients, patients with iodine deficiency and patients with a history of neck irradiation. Uncommonly, a thyroid nodule can cause local compression or hyperthyroidism and so it should be treated accordingly. Yet the clinical importance of thyroid nodules lies in the detection of malignancy, and malignancy comprises approximately 5% of all thyroid nodules irrespective of the size (7). The risk factors associated with an increased likelihood of a malignancy in thyroid nodules include a previous history of irradiation, a family history of medullary thyroid carcinoma or multiple endocrine neoplasia (MEN) type II, patients who are younger than 20 years or older than 60 years, male patients, rapid growth of a nodule, a nodule with a firm and hard consistency, an inconspicuous margin of the nodule on palpation, the presence of enlarged cervical lymph nodes and the presence of a fixed nodule (4, 7, 8).
Among the modern imaging modalities, high-resolution US is the most sensitive diagnostic modality for the detection of the thyroid nodules and it is necessary to perform US for the nodules found after palpation (8). In addition, US can evaluate the size and characteristic of nonpalpable nodules, it can guide FNA for thyroid nodules and it can diagnose lymph node metastasis. Although thyroid US has been regarded as the mainstay for the management of the thyroid nodules, there has been no clear consensus on the US-based management such as follow-up for thyroid US and the selection of a nodule for FNA biopsies, as well as the standardized terminology for thyroid US. There are many different guidelines and recommendations for the management of thyroid nodules detected on US, and these recommendations and guidelines have been described by different organizations (4, 7, 8).
The thyroid study group of the Korean Society of Radiology (TSGKSR) organized a task force group in 2005 and the task force members undertook a complete literature review in 2006 and 2009. The relevant articles from 1985 to 2009 were collected by searching MEDLINE using the following search terms: thyroid nodule, thyroid malignancy, thyroid carcinoma, US, aspiration biopsy, biopsy and follow-up.
Since the TSGKSR first organized the taskforce team to provide recommendations for thyroid US and to undertake a multicenter study (9), the thyroid study group published its recommendations for the US management of thyroid nodules in 2006 (10) and the group revised the recommendations in 2009. Meanwhile, the TSGKSR has been transformed into the Korean Society of Thyroid Radiology (KSThR). By the inclusion of new references (up to May 2010), we provide here this article for any radiologists who perform thyroid US. We have reviewed the standardized terminology for thyroid US as proposed by our task force, the US findings of thyroid nodules and the strategy for US follow-up and USFNA biopsies. We also discuss the current issues for the role of US screening for thyroid nodules.
The size of a thyroid nodule is not helpful for distinguishing a malignant nodule from a benign nodule. The nodule size should be precisely documented for the purpose of follow-up. Although malignancy is believed to grow more prominently than benignancy, even benign nodules can grow with time and about 90% of benign nodules have demonstrated an increase in volume by 15% over a 5-year follow-up period (11, 12). Cystic nodule showed slower growth than did solid nodule (13). The rapid growth of thyroid nodules can be seen for anaplastic thyroid carcinoma, lymphoma, sarcoma and rarely for high-grade carcinoma (14).
Although in principle the size of thyroid nodules should be measured in all three dimensions, only the maximal diameter of the nodule can be measured and documented. When measuring the nodule size, it is advisable to locate the calipers at the outer margin of the halo of the nodule (4).
There has been no clear consensus on the definition of nodule growth. According to the American Thyroid Association (ATA) guideline, a reasonable definition of growth is a 20% increase in the nodule diameter with a minimum increase in two or more dimensions of at least 2 mm, which is roughly a 50% increase in volume (8). Some groups prefer a 15% increase in the nodule volume as a definition of nodule growth (13, 15). Yet substantial interobserver bias has previously been observed, and especially for less than a 50% volume increase in small nodules (16).
Accordingly, we recommend the definition of nodule growth as a 20% increase in the nodule diameter or a 50% increase in the nodule volume.
Although a mainly cystic nodule is rare in thyroid carcinoma, a cystic component is found in 13-26% of all thyroid carcinomas (17, 18). Approximately 5% of all partially cystic nodules have been reported to be malignant in a recent study (19). In this case, the presence of a solid component with vascularity, an eccentric location of the solid portion or microcalcification may suggest malignant nodule and especially papillary thyroid carcinoma (17, 19, 20). A nodule with multiple microcystic spaces separated by thin septae or intervening isoechoic parenchyma (a 'spongiform' appearance) is regarded as a benign nodule with a specificity of 99.7-100% (9, 21, 22).
We suggest the following terminology for the internal content of a nodule. The internal content of a nodule is categorized in terms of the ratio of the cystic portion to the solid portion in the nodule: solid (≤ 10% of the cystic portion), predominantly solid (> 10% of the cystic portion and ≤ 50% of the cystic portion), predominantly cystic (> 50% of the cystic portion and ≤ 90% of the cystic portion) and cystic (> 90% of the cystic portion) (9) (Fig. 1). The definition of a spongiform appearance is the aggregation of multiple microcystic components in more than 50% of the volume of the nodule (9) (Fig. 2).
The shape of a nodule has gained diagnostic importance for the differentiation of benign and malignant nodules since this was first described in a study by Kim et al. (23). Kim and colleagues reported that a taller-than-wide shape showed a specificity of 93% for the diagnosis of malignant nodules. In a larger multicenter study, a taller-than-wide shape was shown to be highly suggestive of a malignancy, with a specificity of 89% and a positive predictive value of 86% (9).These findings reflect that malignant nodules grow across the normal tissue plane in a centrifugal way, while benign nodules grow along the tissue plane in a parallel fashion (23-25). A nodule with an irregular shape is often seen in benign conditions such as focal thyroiditis as well as in malignant conditions (9). We suggest that the shape of a nodule is categorized as follows: ovoid to round (when the anteroposterior diameter of a nodule is equal to or less than its transverse diameter on a transverse or longitudinal plane), taller-than-wide (when the anteroposterior diameter of a nodule is longer than its transverse diameter on a transverse or longitudinal plane) or irregular (when a nodule is neither ovoid to round nor taller-than-wide) (Fig. 3).
Earlier studies have reported that both a spiculated or microlobulated margin and an ill-defined margin are suggestive of malignancy (23, 26). With the development of high-frequency transducer US techniques, a previously described ill-defined margin could actually be a spiculated and jagged edge with sharp demarcation (a spiculated or microlobulated margin) or a poorly defined margin in which the tumor cannot be differentiated from the normal parenchyma (an ill-defined margin) (9). When the marginal tumor infiltration is minimal, it can be seen as an ill-defined margin. In addition, benign thyroid nodules are known to be incompletely encapsulated and poorly marginated and they can merge with normal tissue (27). Therefore, an ill-defined margin is a nonspecificfinding that is seen for both benign and malignant nodules. In contrast, a spiculated margin is a highly suggestive finding of malignancy with a specificity of 92% and a positive predictive value of 81% (9).
Accordingly, we suggest that the margin of a nodule is categorized as follows: smooth, spiculated/microlobulated or ill-defined (Fig. 4).
In terms of echogenicity, a solid component must be considered. When a solid component is heterogeneous, the nodular echogenicity is defined by that of the majority of the nodule. Marked hypoechogenicity is highly specific for malignant nodule with a specificity of 92-94% (9, 23). Although the parenchymal echogenicity of a thyroid gland can vary among individuals, it is used as a reference for nodule echogenicity. Another reference to define nodule echogenicity is the strap muscles with low-echogenicity such as the sternothyroid muscle, the sternothyroid muscle and the sternocleidomastoid muscles (9, 23).
We suggest that nodule echogenicity is categorized according to the relative echogenicity compared to that of a reference as follows. Nodule echogenicity includes marked hypoechoic (when a nodule is hypoechoic relative to the adjacent strap muscle), hypoechoic (when a nodule is hypoechoic relative to the thyroid parenchyma), isoechoic (when a nodule has the same echogenicity as that of the thyroid parenchyma) and hyperechoic (when a nodule is echogenic relative to the thyroid parenchyma) (Fig. 5).
Calcifications can be seen in both benign and malignant nodules. Calcifications may be microcalcification, coarse or macrocalcification or peripheral or rim calcifications in thyroid nodules. Pathologically, microcalcification is a psammoma body that is comprised of 10-100 µm round, laminar, crystalline, calcific deposits, which is very specific for thyroid carcinoma, and especially for papillary thyroid carcinoma.
Microcalcifications on US are findings that are highly suggestive for malignancy with a specificity of 86-95% and a positive predictive value of 42-94% (9, 23, 26, 28, 29). Large and irregular shaped dystrophic calcifications may develop secondarily due to tissue necrosis and these calcifications can be seen in both benign and malignant nodules. A solid nodule with macrocalcification larger than 1 mm suggests the presence of a malignancy rather than a benign nodule (9). The meaning of peripheral, eggshell or rim calcification is still being debated for making the differentiation between benign and malignant nodules. Recent reports have found that when a nodule has eggshell or rim calcifications, a hypoechoic halo and/or disruption of eggshell calcifications, these are findings that suggest malignancy (9, 30-32).
On US, a calcification is defined as a prominent echogenic focus with or without posterior shadowing. The absence of posterior shadowing does not rule out the possibility of calcification since some calcifications are too small to produce posterior shadowing. When punctuate echogenic foci are accompanied by reverberation artifacts, they should be due to colloid materials and they can be easily differentiated from calcification on real-time US.
We suggest that calcification is categorized with respect to its size as follows. Calcifications include microcalcifications (when there are tiny, punctuate echogenic foci of 1 mm or less either with or without posterior shadowing), macrocalcifications (when punctuate echogenic foci are larger than 1 mm in size) and rim calcifications (when a nodule has peripheral curvilinear or eggshell calcification) (Fig. 6).
Extracapsular extension is observed in 36% of all thyroid carcinomas at surgery (26). Aggressive local invasion is relatively common in anaplastic carcinoma, lymphoma and sarcoma. Radiologist should observe whether a nodule crosses the thyroid capsule and invades the adjacent structure such as the trachea, esophagus and thyroid cartilage.
The echotexture of a nodule may be homogeneous or heterogeneous. Echotexture is not a helpful finding in distinguishing malignant nodules from benign nodules (9).
Sometimes a nodule show an accompanying hypoechoic thin or thick halo. A halo or hypoechoic rim surrounding a nodule is comprised of a pseudocapsule that is caused by fibrous connective tissue, compressed thyroid tissue and chronic inflammatory change (33). Although a completely even halo is a finding suggestive of benign nodule with a specificity of 95% in one study (34), more than a half of benign nodules lack a halo (18, 33). On the other hand, 10-24% of all papillary carcinomas have a complete or incomplete halo (14, 17, 18, 34).
Color Doppler US or power Doppler US can be used for the evaluation of the intratumoral vascularity of thyroid nodules. Although intratumoral hypervascularity is observed in 69-74% of thyroid carcinomas, it is a nonspecific finding (17). Though perinodular flow is mainly a characteristic finding for benign nodules, it is observed in 22% of malignant nodules (17). According to several recent studies, the resistive index, the maximal systolic velocity and the vascularity pattern on Doppler US did not help to differentiate benign and malignant nodules (35, 36). Therefore, we do not recommend the routine use of color Doppler and power Doppler US for thyroid nodules.
US elastography is a new technique to measure the elasticity of tissue. The tissue of carcinoma is harder and firmer than that of the normal thyroid parenchyma or a benign nodule. Elastography quantifies the firmness of the tissue and this is visualized as an elastography map. The strain index on elastography has been suggested as a good predictive factor for malignant thyroid nodules (37) (Fig. 7).
The known US findings for malignant nodules are microcalcifications, the presence of hypoechoic nodule, an irregular margin, loss of the halo and the presence of a solid nodule as well as intratumoral vascularity (5, 24, 26, 28, 29, 38-40). However, the different use of terminology, the variable sample size of the previously published data, the use of US instruments with different qualities and the different range of experience of radiologists and even diagnostic overlap of these findings cause variable results of the diagnostic accuracy (4, 23, 26, 28, 29, 41).
In the multicenter retrospective study that applied standardized terminology for US findings as proposed earlier in this report (9), the significant findings for malignant nodules were a taller than wide shape (sensitivity, 40%; specificity, 91%), a spiculated margin (sensitivity, 48%; specificity, 92%), marked hypoechogenicity (sensitivity, 41%; specificity, 92%), microcalcification (sensitivity, 44%; specificity, 91%), and macrocalcification (sensitivity, 10%; specificity, 96%). The US findings for benign nodules were isoechogenicity (sensitivity, 57%; specificity, 88%) and a spongiform appearance (sensitivity, 10%; specificity, 100%). Although hypoechogenicity is a suggestive finding for being malignancy in many reports (4, 23, 26, 39), marked hypoechogenicity is a more specific and more reliable criterion for a malignant nodule (9, 23). In terms of the nodule size, a lower frequency of microcalcification in microcarcinomas causes a lower sensitivity and this suggests that microcalcification is not a major predictor of malignancy in nodules 1 cm or smaller. Although other findings such as marked hypoechogenicity, a taller-than-wide shape and a spiculated margin are slightly more frequent in subcentimeter malignant nodules, and they are also more frequent in subcentimeter benign nodules than in the larger counterparts. Accordingly, the false positive rate of depiction of a malignant nodule could be increased in smaller nodules (9, 42).
Although rim calcification itself was not helpful for making a US diagnosis in a multicenter study, recent studies suggest that the presence of a hypoechoic halo and disruption of rim calcification may be useful sonographic predictors of malignancy (32). In addition, according to previous studies, the findings of a complete cystic lesion and a cystic lesion containing comet tail artifacts are very specific for benignancy (43). Therefore, we suggest the US criteria for benign and malignant thyroid nodules as is shown in Figure 8. We divided thyroid nodules into three categories: suspicious malignant nodules, probably benign nodules and indeterminate nodules. A taller-than-wide shape, a spiculated margin, marked hypoechogenicity, microcalcifications and macrocalcifications are suggestive findings for malignancy. The presence of at least one of the findings for malignancy defines a nodule as a suspicious malignant nodule. In contrast, a simple cyst, a predominantly cystic or cystic nodule with reverberating artifacts and a nodule with a spongiform appearance (especially with intervening isoechoic parenchyma) are defined as probably benign nodules. Although a cystic nodule with more than a 90% cystic component is very rare for a thyroid malignancy, a mural solid component within the cystic nodule may be papillary thyroid carcinoma (43). Therefore, a small solid component in a predominantly cystic or cystic nodule should be carefully examined and it should be aspirated in the case with the presence of a suspicious malignant feature.
Indeterminate nodules include nodules having US findings with neither malignant nor benign features. The US findings for an indeterminate nodule include isoechogenicity, hypoechogenicity, and hyperechogenicity, an ovoid-to-round or irregular shape, a smooth or ill-defined margin and a rim calcification. Although, isoechogenicity is more suggestive of benignancy, 14% of the isoechoic nodules were malignant in one study (9). Although rim calcification is classified as an indeterminate factor for malignancy, the presence of a hypoechoic halo and rim disruption is more suggestive of malignancy (32). The indeterminate category is currently a term for all nodules that are without clear evidence of being benign and malignant. The criteria for suspicious malignant nodules are identical to the US findings for papillary carcinoma, which comprises most thyroid carcinomas. The US findings for medullary carcinoma are almost the same as those for papillary carcinoma (44). Yet these criteria have limited value in diagnosing thyroid carcinomas other than a subtype of papillary carcinoma. Relatively uncommon FTC and other histologic types of thyroid carcinoma can be eliminated by the use of these criteria (45, 46). Along with an accumulation of evidence from further studies on thyroid US, the indeterminate category can be subclassified into truly benign nodule or adenoma or malignant nodule.
Our task force members established recommendations on the indications for US-guided fine-needle aspiration (USFNA) biopsy and follow-up for thyroid nodules first in 2006 (10) and these were revised in 2009. In our recommendations, whether or not to perform a USFNA biopsy depends on the US findings of a nodule (Fig. 8).
When a single nodule is found on thyroid US, the presence of at least one malignant US findings necessitates USFNA regardless of the size of the nodule. Evidence has shown that the mortality and rate of recurrence is directly proportional to the size of a thyroid tumor (47, 48). However, even a micropapillary thyroid carcinoma has a substantial number of lymph node metastases (8-50%) and a recurrence rate from 1% up to approximately 7% (49-53). The ATA guideline recommends that a subcentimeter nodule should be subjected to a biopsy only if the nodule has a suspicious finding or the patient has a personal history of radiation exposure or familial thyroid cancer (8). Among the subcentimeter cancers, a carcinoma smaller than 5 mm has a better survival rate and a better recurrence rate at 5 year (less than 3% versus 14% for carcinoma that is 6-10 mm in diameter) (54). Regarding the size issue, recent studies have recommended not to biopsy nodules smaller than 5 mm in size because of a high rate of false positive US findings as well as a high rate of inadequate cytology (55, 56). We recommend performing FNA for a nodule of any size that has suspicious malignant findings if FNA is feasible and a nodule is larger than 5 mm in size. For a nodule smaller than 5 mm, selective FNA can be done according to patient's risk factors and the experience of the radiologists. This recommendation relies on the fact that there is still debate concerning the fate and prognosis of microcarcinomas, as was described above.
If a nodule has indeterminate findings on US and it is larger than 1 cm in diameter, then performing FNA is recommended due to the fact that the possibility of malignancy cannot be excluded. If a nodule has indeterminate findings and it is 1 cm or less in size, then an FNA biopsy is not necessary and follow-up US would suffice. If a benign appearing nodule is larger than 1 cm, then we recommend performing follow-up US in two years and thereafter at 3-5 year intervals. If a benign appearing nodule (i.e., a spongiform nodule) is larger than 2 cm, then selective FNA biopsy can be done. Neither FNA nor follow up US is necessary for a spongiform nodule and a benign appearing nodule 1 cm or less in diameter.
When multiple nodules are found on US, not all of the nodules have to be biopsied. The risk of malignancy for patients with multiple thyroid nodules is not greatly different from that for patients with a single thyroid nodule (8, 26). According to the ATA guideline (8), in the presence of two or more nodules 1-1.5 cm or more in size, a FNA biopsy is recommended for nodules with suspicious US findings. If none of the nodules has suspicious US findings, then FNA should be done for the largest one. Multifocality and bilaterality is not uncommon and even in thyroid microcarcinomas (49). Furthermore, some investigators believe multifocality and bilaterality are linked to higher recurrence and higher mortality (48, 50). Therefore, multiple and bilateral nodules should not be regarded as a multinodular goiter consisting of benign nodules.
In the case of multiple nodules of the thyroid, we choose the nodules to be biopsied according to the US findings. We recommend aspirating one or more nodules that meet the US criteria of a nodule, but not to depend on the size criteria. We recommend aspirating at least one nodule for each lobe and at least one nodule (the largest) among multiple nodules that have similar US findings. A nodule should not be chosen for a biopsy only on the size criteria alone.
Some thyroid nodules may grow steadily as seen on follow-up US, even though they were diagnosed as being benign on the previous cytology. In these cases, a decision should be made on whether or not to perform a biopsy. The rate of nodule growth on US cannot distinguish benign from malignant nodules (57). In one study of 420 benign nodules, one-third of benign nodules showed growth of a 15-30% increase in volume, one-third of the benign nodules showed no change in growth and one-third showed a decrease in size (58). The measurement of small nodule reportedly is not reliable as substantial interobserver bias has been observed in the measurement of small nodules, and especially for less than a 50% volume increase (16). Despite the debate concerning significant size change, we advise to selectively biopsy a growing nodule according to the size change criteria adopted from the ATA guideline (8). We do recommend an FNA biopsy be performed if an indeterminate nodule is growing. If a benign nodule is growing, then we do not recommend performing an immediate FNA biopsy in every incidence. At least one more follow-up US exams can be selectively advised.
When a malignant lymph node is suspected, it is necessary to biopsy any suspicious lymph node in the lateral neck area as well as a thyroid nodule regardless of the nodule's size and features. While central neck dissection is performed in almost all the patients with thyroid papillary carcinoma, lateral neck dissection (levels II-V) is selectively done for patients who have a preoperative diagnosis of lymphatic metastasis. Therefore, USFNA for suspicious lymph nodes in the lateral neck area (levels II-V) is important for making decisions about surgical management (8).
When the initial US findings are probably benign, a USFNA biopsy is not necessary and follow-up US or clinical observation is advisory for a nodule's change. Among the probably benign nodules on US, a complete cystic or cystic nodule with reverberating artifacts and spongiform nodules can be placed under clinical observation alone. In contrast, the patients with a probably benign nodule larger than 1 cm are recommended to undergo follow-up by US in two years and thereafter at a 3-5 year interval.
When the initial US findings are suspicious for malignant or they are indeterminate, the fate of thyroid nodules is dependent on the adequately classified cytology results (59). If the cytology of the nodule results in nondiagnostic aspirates, then the presence of malignant US findings determines the next step for the management of the nodule. Non-diagnostic cytology results occur in 5-10% of cases under US guidance and these non-diagnostic cases are due to the operator's inexperience, aspiration of cystic fluid and the presence of a bloody aspirate (38). Five percent of nodules with nondiagnostic cytology after an initial biopsy are eventually diagnosed as malignant and approximately 18% of malignant nodules are diagnosed by at least two aspiration cytology examinations (60, 61). Therefore, thyroid nodules with non-diagnostic cytology and malignant US findings should be followed by US-FNA biopsy at a 3-6 month interval. When a nodule does not have any malignant US findings, but it has non-diagnostic cytology, it is recommended that the nodule should be followed up by US-FNA biopsy in 6-12 months.
When the cytology result for a nodule is malignant, the patient should undergo surgery and follow-up by US (7, 8). When the cytology results of thyroid nodules are indeterminate (suspicious for a papillary carcinoma, atypical cells, follicular lesion or follicular neoplasm), the subtype of the indeterminate cytology and the presence of malignant US findings determines the next step for the nodules' management. An indeterminate cytology finding, although the cytology results can vary as determined at different institutions, is responsible for approximately 15-30% of all the fine needle aspiration cytologies. The cytology results of a suspicious for papillary carcinoma or Hurthle cell neoplasm necessitates surgery (lobectomy or total thyroidectomy) followed by US. When the cytology of a nodule is indicative of a follicular neoplasm, it is recommended to consider surgery first although repeated US-FNA biopsies are preferable in certain situations. When the cytology of a nodule is a follicular lesion of undetermined significance or atypia of undetermined significance (59), a nodule 1 cm or more is recommended to undergo a repeated US-FNA biopsy in 6-12 months in the case of malignant US findings, while a nodule without malignant US findings can be subjected to a repeated US-FNA in 1-1.5 years.
When the cytology of a nodule is indicative of being benign, the follow-up strategy is as follows and according to the US findings. A nodule with malignant US findings is recommended to undergo repeat US-FNA in 6-12 months, while a nodule without malignant findings is recommended to undergo repeat US in one year or to repeat US-FNA selectively. Since the false-negative rate of USFNA is low but not negligible, it is reasonable to repeat US-FNA in certain conditions, and especially for thyroid nodules with malignant US findings (62). A nodule with a benign cytology and that has been subjected to at least two USFNA biopsies is regarded as a benign nodule and it can be followed up in 3-5 years.
The role of a screening test for thyroid nodules is limited. Because of the very high prevalence of thyroid nodules and the very good prognosis and survival rate, the current consensus is that a screening test for thyroid malignancy cannot be justified (7). As smaller malignant nodules can be detected on thyroid US, the survival rate and prognosis may improve regardless of the actual effect of the treatments, and even with an increasing prevalence of disease (54, 63). Thyroid cancer detected by the use of an early screening test may tend to progress less rapidly than clinically detected disease. There may be cases that would regress, remain stable or progress too slowly to become clinically apparent during the lifetime of the patient (63).
However, a screening test can be justified in high-risk groups such as patients with a history of familial thyroid carcinoma, a history of MEN or a history of childhood irradiation of the head and neck area.
US for thyroid nodules is the most sensitive diagnostic modality for making the diagnosis of thyroid carcinoma and this modality provides valuable guidance to perform an aspiration biopsy and follow-up. On the US of thyroid nodule, the size of the nodule, the internal texture, the shape, the echogenicity, the margin, the presence of calcification and the presence of adjacent structures should be carefully scrutinized. The findings for a suspicious malignant nodule include a taller-than-wide shape, a spiculated or microlobulated margin, marked hypoechogenicity, microcalcifications and macrocalcifications. Presence of at least one of the malignant US findings suggests the presence of a malignancy. According to these findings and the resultant category of a nodule, the nodule should be aspirated or followed-up with US, or it should remain under clinical observation.
Figures and Tables
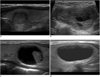 | Fig. 1Internal content of thyroid nodules.
A. Solid B. Predominantly solid C. Predominantly cystic D. Cystic
|
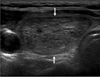 | Fig. 2US findings of spongiform appearance are shown
Transverse US image of benign nodular hyperplasia shows well-defined smooth isoechoic mass with a spongiform appearance (arrows).
|
 | Fig. 3Shape of thyroid nodules. Corresponding schematic drawings are shown in upper panel.
A. Ovoid-to-round shape B. Taller-than-wide shape C. Irregular shape
|
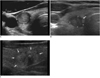 | Fig. 4Margin of thyroid nodules.
A. Smooth margin B. Spiculated or microlobulated margin (arrow) C. Ill-defined margin (arrows)
|
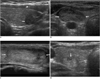 | Fig. 5Echogenicity of thyroid nodules.
A. Marked hypoechogenicity of nodule is shown. Note more hypoechoic nature of nodule (arrow) as compared to that of strap muscles (asterisk). B. Hypoechogenicity of nodule (arrows) C. Isoechogenicity of nodule (arrows) D. Hyperechogenicity of nodule (arrow)
|
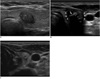 | Fig. 6Calcifications of thyroid nodules.
A. Microcalcification within nodule as echogenic focus B. Macrocalcification (arrow) in center of nodule (white triangles) C. Rim calcification in small nodule (calipers).
|
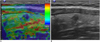 | Fig. 7US elastography of thyroid nodules.
A. Elastography shows nodule with hard consistency as blue relative to green background. B. Longitudinal US image shows same nodule with suspicious malignant US features. Nodule was proven to be papillary carcinoma.
|
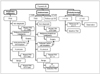 | Fig. 8Flowchart for strategy for follow-up US and US-guided fine needle aspiration (USFNA) biopsy according to US findings and cytology results of thyroid nodules.
Dotted arrow means that surgery is not strongly recommended, but it can be considered according to individual situations. AUS = atypia of undetermined significance, FLUS = follicular lesion of undetermined significance.
|
Acknowledgment
KSThR Taskforce on Thyroid Nodule members are as following in alphabetical order: Jung Hwan Baek, MD (University of Ulsan College of Medicine), So Lyung Jung, MD (College of Medicine, The Catholic University of Korea), Dong Wook Kim, MD (Inje University College of Medicine), Eun Kyung Kim, MD (Yonsei University College of Medicine), Ji Young Kim, MD (College of Medicine, The Catholic University of Korea), Ji Hoon Kim, MD (Seoul National University College of Medicine), Jin Young Kwak, MD (Yonsei University College of Medicine), Jeong Hyun Lee, MD (University of Ulsan College of Medicine), Joon Hyung Lee, MD (Dong-A University College of Medicine), Young Hen Lee, MD (Korea University School of Medicine), Won-Jin Moon, MD (Konkuk University School of Medicine), Dong Gyu Na, MD (Human Medical Imaging & Intervention Center), Jeong Seon Park, MD (Hanyang University College of Medicine), Sun Won Park, MD (Seoul National University College of Medicine), Jung Hee Shin, MD (Sungkyunkwan University School of Medicine).
The authors sincerely thank Ji Hoon Kim, MD and Jung Hee Shin, MD for their most valuable advice and support in developing recommendations. In addition, the authors sincerely appreciate Jinna Kim, MD for her contribution in organizing the former Thyroid Study Group of Korean Society of Radiology (TSGKSR), which was a predecessor of KSThR.
References
1. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer. 1985. 56:531–538.
2. Brander A, Viikinkoski P, Nickels J, Kivisaari L. Thyroid gland: US screening in a random adult population. Radiology. 1991. 181:683–687.
3. Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997. 126:226–231.
4. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005. 237:794–800.
5. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004. 60:21–28.
6. 2005 annual report of the Korea central cancer registry [www document]. National Cancer Information Center K. last accessed; Oct 2008. Available at : http://www.cancer.go.kr.
7. Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006. 12:63–102.
8. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
9. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.
10. Moon WJ, Na DG, Jung SL, Lee JH, Kim J, Kim HS, et al. Recommendations for ultrasound-based management of thyroid nodules. 62nd Scientific Assembly of the Korean Radiological Society. 2006. Seoul: The Korean Radiological Society.
11. Brander AE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000. 215:801–806.
12. Kuma K, Matsuzuka F, Yokozawa T, Miyauchi A, Sugawara M. Fate of untreated benign thyroid nodules: results of long-term follow-up. World J Surg. 1994. 18:495–498.
13. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003. 138:315–318.
14. Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US features of thyroid malignancy: pearls and pitfalls. Radiographics. 2007. 27:847–860.
15. Papini E, Petrucci L, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, et al. Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab. 1998. 83:780–783.
16. Brauer VF, Eder P, Miehle K, Wiesner TD, Hasenclever H, Paschke R. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid. 2005. 15:1169–1175.
17. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003. 22:1083–1090.
18. Watters DA, Ahuja AT, Evans RM, Chick W, King WW, Metreweli C, et al. Role of ultrasound in the management of thyroid nodules. Am J Surg. 1992. 164:654–657.
19. Lee MJ, Kim EK, Kwak JY, Kim MJ. Partially cystic thyroid nodules on ultrasound: probability of malignancy and sonographic differentiation. Thyroid. 2009. 19:341–346.
20. Hatabu H, Kasagi K, Yamamoto K, Iida Y, Misaki T, Hidaka A, et al. Cystic papillary carcinoma of the thyroid gland: a new sonographic sign. Clin Radiol. 1991. 43:121–124.
21. Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009. 193:207–213.
22. Moon WJ, Kwag HJ, Na DG. Are there any specific ultrasound findings of nodular hyperplasia ("leave me alone" lesion) to differentiate it from follicular adenoma? Acta Radiol. 2009. 50:383–388.
23. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002. 178:687–691.
24. Alexander EK, Marqusee E, Orcutt J, Benson CB, Frates MC, Doubilet PM, et al. Thyroid nodule shape and prediction of malignancy. Thyroid. 2004. 14:953–958.
25. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.
26. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 87:1941–1946.
27. Reading CC, Charboneau JW, Hay ID, Sebo TJ. Sonography of thyroid nodules: a "classic pattern" diagnostic approach. Ultrasound Q. 2005. 21:157–165.
28. Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck. 2002. 24:651–655.
29. Peccin S, de Castsro JA, Furlanetto TW, Furtado AP, Brasil BA, Czepielewski MA. Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest. 2002. 25:39–43.
30. Kwak MS, Baek JH, Kim YS, Jeong HJ. Patterns and significance of peripheral calcifications of thyroid tumors seen on ultrasound. J Korean Radiol Soc. 2005. 53:401–405.
31. Yoon DY, Lee JW, Chang SK, Choi CS, Yun EJ, Seo YL, et al. Peripheral calcification in thyroid nodules: ultrasonographic features and prediction of malignancy. J Ultrasound Med. 2007. 26:1349–1355.
32. Kim BM, Kim MJ, Kim EK, Kwak JY, Hong SW, Son EJ, et al. Sonographic differentiation of thyroid nodules with eggshell calcifications. J Ultrasound Med. 2008. 27:1425–1430.
33. Propper RA, Skolnick ML, Weinstein BJ, Dekker A. The nonspecificity of the thyroid halo sign. J Clin Ultrasound. 1980. 8:129–132.
34. Lu C, Chang TC, Hsiao YL, Kuo MS. Ultrasonographic findings of papillary thyroid carcinoma and their relation to pathologic changes. J Formos Med Assoc. 1994. 93:933–938.
35. Tamsel S, Demirpolat G, Erdogan M, Nart D, Karadeniz M, Uluer H, et al. Power Doppler US patterns of vascularity and spectral Doppler US parameters in predicting malignancy in thyroid nodules. Clin Radiol. 2007. 62:245–251.
36. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010. 255:260–269.
37. Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005. 237:202–211.
38. Kim SJ, Kim EK, Park CS, Chung WY, Oh KK, Yoo HS. Ultrasound-guided fine-needle aspiration biopsy in nonpalpable thyroid nodules: is it useful in infracentimetric nodules? Yonsei Med J. 2003. 44:635–640.
39. Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003. 22:1027–1031.
40. Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004. 23:1455–1464.
41. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003. 22:127–131.
42. Popowicz B, Klencki M, Lewiński A, Słowińska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule's size. Eur J Endocrinol. 2009. 161:103–111.
43. Ahuja A, Chick W, King W, Metreweli C. Clinical significance of the comet-tail artifact in thyroid ultrasound. J Clin Ultrasound. 1996. 24:129–133.
44. Kim SH, Kim BS, Jung SL, Lee JW, Yang PS, Kang BJ, et al. Ultrasonographic findings of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Korean J Radiol. 2009. 10:101–105.
45. Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007. 8:192–197.
46. Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, et al. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009. 28:1685–1692.
47. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993. 328:553–559.
48. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–428.
49. Pazaitou-Panayiotou K, Capezzone M, Pacini F. Clinical features and therapeutic implication of papillary thyroid microcarcinoma. Thyroid. 2007. 17:1085–1092.
50. Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998. 83:553–559.
51. Chow SM, Law SC, Au SK, Mang O, Yau S, Yuen KT, et al. Changes in clinical presentation, management and outcome in 1348 patients with differentiated thyroid carcinoma: experience in a single institute in Hong Kong, 1960-2000. Clin Oncol (R Coll Radiol). 2003. 15:329–336.
52. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003. 13:381–387.
53. Noguchi S, Yamashita H, Murakami N, Nakayama I, Toda M, Kawamoto H. Small carcinomas of the thyroid. A long-term follow-up of 867 patients. Arch Surg. 1996. 131:187–191.
54. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008. 32:747–753.
55. Kim DW, Lee EJ, Kim SH, Kim TH, Lee SH, Kim DH, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules: comparison in efficacy according to nodule size. Thyroid. 2009. 19:27–31.
56. Mazzaferri EL, Sipos J. Should all patients with subcentimeter thyroid nodules undergo fine-needle aspiration biopsy and preoperative neck ultrasonography to define the extent of tumor invasion? Thyroid. 2008. 18:597–602.
57. Asanuma K, Kobayashi S, Shingu K, Hama Y, Yokoyama S, Fujimori M, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001. 167:102–105.
58. Erdogan MF, Gursoy A, Erdogan G. Natural course of benign thyroid nodules in a moderately iodine-deficient area. Clin Endocrinol (Oxf). 2006. 65:767–771.
59. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009. 19:1159–1165.
60. Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasoundguided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002. 87:4924–4927.
61. Ogawa Y, Kato Y, Ikeda K, Aya M, Ogisawa K, Kitani K, et al. The value of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: an assessment of its diagnostic potential and pitfalls. Surg Today. 2001. 31:97–101.
62. Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010. 254:292–300.
63. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993. 328:1237–1243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download