Abstract
A lymphoepithelioma-like carcinoma, characterized by a carcinoma with heavy lymphocyte infiltration, is one of the histological patterns observed in patients with Epstein-Barr virus (EBV)-associated gastric carcinoma. Less than half of invasive carcinomas with lymphoepithelioma-like histology can grow to make a submucosal mass. These tumors generally have a better prognosis than conventional adenocarcinomas. We report a case of an EBV-associated lymphoepithelioma-like gastric carcinoma that presented as a submucosal mass on multi-detector (MD) CT and correlate them with the pathology.
Since Eptein-Barr virus (EBV) DNA was first detected in a patient with undifferentiated lymphoepithelioma-like gastric carcinoma, EBV is known to be causally associated with gastric carcinomas in a small percentage of patients (1-5). Lymphoepithelioma-like carcinoma, pathologically characterized by a tumor that consists of a lymphoid stroma and small nests of cancer cells, is one of the histological patterns observed in patients with EBV-associated gastric carcinomas (1-3, 5, 6). The majority of these tumors arising in the stomach have been reported to be related to EBV infections (2-4).
Sometimes, lymphoepithelioma-like gastric carcinomas invaded the submucosa, forming a submucosal nodule that consists of cancer cells admixed with lymphoid stroma (1, 7). The submucosal mass lesion, one of the various appearances of lymphoepithelioma-like gastric carcinoma, can be misdiagnosed as submucosal tumors, such as a gastrointestinal stromal tumor (GIST), carcinoid tumor, or glomus tumor. Here, we describe the multi-detector (MD) CT findings of an EBV-associated lymphoepithelioma-like gastric carcinoma presenting as a submucosal mass.
A 46-year-old man was transferred to our hospital for further evaluation and management of a gastric lesion that had been detected by gastroscopy during a medical checkup at a local clinic. The gastroscopy showed an approximately 2.5-cm sized submucosal mass with two active ulcers on the mucosal surface (Fig. 1A). The diagnostic impression of the referring physician in the local clinic was a GIST or lymphoma. After a gastroscopic biopsy of the mucosal ulcer, the gastric lesion was diagnosed pathologically as an adenocarcinoma at this clinic.
A contrast-enhanced CT of the abdomen using a 64-MDCT scanner (LightSpeed VCT, GE Healthcare, Milwaukee, WI) was performed at our hospital. The CT images revealed a 1.5 × 2.5-cm ovoid mass with contrast enhancement at the lesser curvature wall of the gastric high body near the cardia (Fig. 1B, C). Because intact overlying mucosa was identified, the mass was initially thought to be a submucosal tumor such as a GIST. There was no evidence of perigastric infiltration, enlarged lymph nodes, or distant metastasis on CT. After reviewing the clinical information of the biopsy-proven adenocarcinoma, a gastric carcinoma was included in the differential diagnosis on the radiological report. However, because the enhanced mass in the submucosal layer was not the usual CT finding for a gastric adenocarcinoma, comparison with the clinical findings of gastroscopy and pathology was recommended.
A total gastrectomy was carried out to remove the gastric mass. The resected specimen appeared as a 2.0 × 2.5-cm submucosal mass with small elevated lesions and central ulcerations on the mucosa. On light microscopy, poorly differentiated adenocarcinoma with extensive lymphocytic reaction in the mucosa that invaded the submucosa and subsequently formed a submucosal mass was confirmed (Fig. 1D, E). The tumor invaded the subserosal adipose tissue, and was of the diffuse histological type (Lauren's classification). No lymph node metastasis was present. The presence of EBV in gastric tumor cells was confirmed by performing EBV-encoded RNA-1 (EBER-1) in situ hybridization.
An etiologic association between EBV and gastric carcinoma has been demonstrated by the uniform expression of EBV in all carcinoma cells and its absence in normal epithelium or dysplastic lesions (1, 2). EBV-associated gastric carcinomas have an approximately 9% overall prevalence and, tend to be located in the cardia and body of the stomach when compared with conventional gastric adenocarcinoma (3, 4). These tumors are closely related to postsurgical gastric stump/remnant cancers (3, 4). In addition, approximately 15-25% of EBV-associated gastric carcinomas exhibit the lymphoepithelioma-like pattern, whereas 86-91% of lymphoepithelioma-like gastric carcinomas were EBV positive (3, 4).
Carcinomas with morphologic features similar to undifferentiated nasopharyngeal carcinomas are designated as lymphoepithelioma-like carcinomas and occur in the stomach, salivary gland, lung, and thymus. The pathologic findings indicate that the tumor cells, which are arranged in sheets or nests or as isolated cells, are surrounded by a dense population of non-neoplastic small lymphocytes (6).
EBV-associated gastric carcinomas can present as two histomorphological forms of a lymphoepithelioma-like carcinoma and a glandular adenocarcinoma, and these tumors are characteristically accompanied by prominent lymphocyte infiltration; EBV has been thought of as being the main cause for the lymphocytic response (1, 2, 8, 9). The intramucosal lace pattern with lymphocyte infiltration has been reported to be the most characteristic morphologic feature of EBV-associated gastric carcinomas. In addition, carcinomas with submucosal invasion had a lymphoepithelioma-like pattern of the submucosa in 42% of cases (1). The pathogenesis of the abundant lymphoid stroma in the submucosal layer remains unclear, but it is presumed that carcinomas with a lymphocytic reaction in the mucosa may invade the submucosa, and this lesion in the submucosa then grows due to the factors associated with lymphocytic induction, resulting in the formation of a submucosal mass (1).
Lymphoepithelioma-like gastric carcinomas have a better prognosis than other forms of EBV-associated gastric carcinomas and conventional gastric carcinomas (1, 2, 9, 10). This is because the spread of tumors through the gastric wall and to the lymph nodes or remote organs may be prevented by the abundant lymphocytic reaction; that is, the patient's inflammatory response may contribute to a better prognosis in patients with EBV-associated gastric carcinoma (1, 9).
Despite its distinct clinicopathologic features, lymphoepithelioma-like gastric carcinomas are not familiar to most radiologists. In a CT study including 13 EBV-associated gastric carcinomas, authors concluded that the carcinomas were generally located in the upper gastric region, with a large thickness-to-width ratio, or with a bulky mass projecting from the wall (11). However, the EBV-associated gastric carcinomas had various appearances on CT including focal mucosal thickening, marked wall thickening with contrast enhancement, and bulky mass formation. These appearances on imaging may overlap with cases of early gastric carcinoma, advanced gastric carcinoma, and lymphoma. In another study describing the endoscopic ultrasonographic findings of four EBV-associated early gastric carcinomas, hypoechoic submucosal nodules that corresponded to lymphoid stroma composed of carcinoma cells and infiltrating lymphocytes were observed in three cases that invaded the submucosa (7).
It is difficult to differentiate lymphoepithelioma-like gastric carcinoma presenting as a submucosal mass from other gastric submucosal tumors such as a GIST, neurogenic tumor, and glomus tumor by imaging alone. However, as noted in the case reported here, a discrepancy between imaging findings suggestive of a submucosal tumor and the biopsy results of the adenocarcinoma may suggest the diagnosis present in this case.
Although mucosal ulceration can be present in both EBV-associated gastric carcinomas and submucosal tumors, the ulcerated shape of the advanced EBV-associated lesions has been thought to be related to the superficial depressed shape of the early lesions (12).
In conclusion, the MDCT findings of an EBV-associated lymphoepithelioma-like carcinoma that developed in the gastric body presented as an enhancing submucosal mass with abundant lymphoid stroma admixed with adenocarcinoma. Because the tumors have unique clinicopathologic features, understanding the imaging findings and clinical features of EBV-associated lymphoepithelioma-like carcinomas is important in the preoperative diagnosis and in differentiating this entity from other submucosal tumors.
Figures and Tables
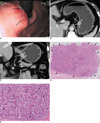
Fig. 1
46-year-old man with Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma.
A. Gastroscopy revealed submucosal mass with two mucosal ulcerations (arrows) in gastric high body near cardia.
B. Contrast-enhanced axial CT scan shows ovoid enhancing mass (arrows) in submucosal layer of gastric high body. Note overlying mucosa with contrast enhancement (arrowheads).
C. Coronal reformatted image shows submucosal mass with contrast enhancement near cardia (arrows). Also note overlying mucosa (arrowheads).
D. Low-power photomicrograph (Hematoxylin & Eosin stain, ×4) shows tumor infiltration in mucosa (arrows), mucosal ulcerations (U) and well-demarcated submucosal mass of lymphoid stroma (arrowheads).
E. High-power photomicrograph (Hematoxylin & Eosin stain, ×200) shows multifocal small nests of poorly differentiated adenocarcinomas (arrows) that are uniformly distributed throughout lymphoid stroma. These findings of pathology were observed in all of mucosal and submucosal layers.
References
1. Arikawa J, Tokunaga M, Satoh E, Tanaka S, Land CE. Morphological characteristics of Epstein-Barr virus-related early gastric carcinoma: a case-control study. Pathol Int. 1997. 47:360–367.
2. Herath CH, Chetty R. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 2008. 132:706–709.
3. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009. 137:824–833.
4. Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009. 24:354–365.
5. Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990. 3:377–380.
6. Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995. 103:308–315.
7. Nishikawa J, Yanai H, Mizugaki Y, Takada K, Tada M, Okita K. Case report: hypoechoic submucosal nodules: a sign of Epstein-Barr virus-associated early gastric cancer. J Gastroenterol Hepatol. 1998. 13:585–590.
8. Shah KM, Young LS. Epstein-Barr virus and carcinogenesis: beyond Burkitt's lymphoma. Clin Microbiol Infect. 2009. 15:982–988.
9. Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010. 139:84–92.
10. Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000. 118:1031–1038.
11. Maeda E, Akahane M, Uozaki H, Kato N, Hayashi N, Fukayama M, et al. CT appearance of Epstein-Barr virus-associated gastric carcinoma. Abdom Imaging. 2009. 34:618–625.
12. Yanai H, Nishikawa J, Mizugaki Y, Shimizu N, Takada K, Matsusaki K, et al. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Gastrointest Endosc. 1997. 45:236–242.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download