Abstract
Objective
The objective of this study was to determine the sequential CT findings of controlled hepatocellular carcinoma (HCC) with main portal vein (MPV) thrombosis with the use of transcatheter arterial chemoembolization and additional intra-arterial cisplatin infusion.
Materials and Methods
From January 2004 to September 2006, 138 patients with HCC invading MPV were referred to the angiography unit of our institution for chemoembolization and additional intra-arterial cisplatin infusion. Until August 2008, seven (5%) of 138 patients were followed-up and found not to have tumor recurrence. CT scans were retrospectively reviewed by two radiologists, focusing on the following parameters: the extent of portal vein thrombosis, the diameter of the affected portal vein, and enhancement of portal vein thrombosis.
Results
The extent of portal vein thrombosis at the initial presentation was variable: left portal vein (LPV) and MPV (n = 1), right portal vein (RPV) and MPV (n = 3), as well as RPV, LPV and MPV (n = 3). The extent and diameter of the affected portal vein decreased during follow-up examinations. In addition, the degree of enhancement for tumor thrombi and serum alpha-feto-protein levels decreased after the transcatheter arterial chemoembolization. Portal vein thrombosis was found to be completely resolved in one patient, whereas residual thrombus without viability was persistent in six patients.
Transcatheter arterial chemoembolization is a widely performed procedure for inoperable hepatocellular carcinomas (HCCs) (1, 2). Recent meta-analysis result has shown that chemoembolization can improve patient survival (3). However, only a few case reports have described HCCs with main portal vein (MPV) invasion that have been cured by chemoembolization (4-6). Moreover, chemoembolizations in patients with MPV tumor thrombosis have the potential risk to cause hepatic insufficiency and the survival benefit of chemoembolization has not been proven (6-8). The use of continuous hepatic arterial infusion chemotherapy, interferon-α-based chemotherapy, and three-dimensional conformal radiotherapy has shown favorable results in patients with portal vein tumor thrombosis (9, 10).
Recently, we have attempted a chemoembolization with additional intra-arterial cisplatin infusion in patients with portal vein tumor thrombosis (11). Seven HCC cases with MPV tumor thrombosis were cured. The goal of this study was to determine the sequential CT findings of the seven patients with controlled HCCs accompanied by MPV thrombosis, which was treated by transarterial chemoembolization and additional intra-arterial cisplatin infusion.
From January 2004 to September 2006, 1,359 patients who were newly diagnosed with HCC were referred to the angiography unit of our institution for chemoembolization. For 138 out of 1,359 patients, MPV tumor thrombosis was evident at initial presentation. Until August 2008, seven patients (5%) have been followed-up without tumor recurrence. The seven patients were all male with an age range of 32-72 years (mean age, 53 years). Six patients were hepatitis B carriers, while one was a hepatitis C carrier. In all cases, a diagnosis of HCC was made based on laboratory testing (e.g., elevated α-fetoprotein levels and expression of viral markers) with typical CT and angiographic findings. All patients had received repeated chemoembolization, and one patient received additional radiation therapy for portal vein thrombosis. The criteria of 'complete remission' are as follows: 1) no enhancement on a portion in the liver, suggesting the presence of a viable tumor as seen on a CT image, 2) no hypervascular staining as detected on angiography, 3) a serum α-fetoprotein level less than 20 ng/mL, 4) no metastasis detected on a CT image. The institutional review board approved this study and patient informed consent was waived due to the retrospective nature of the study.
Every patient had undergone a CT examination every two or three months. The CT examinations were performed using various multi-detector row CT scanners. The scanners included a four-detector row CT scanner (MX 8000, Philips Medical Systems, Cleveland, OH), an eight-detector row CT scanner (Light Speed Ultra, GE Medical Systems, Milwaukee, WI), a 16-detector row CT scanner (Sensation 16, Siemens, Erlangen, Germany), and a 64-detector row CT scanner (Brilliance 64, Philips Medical Systems). All patients who received chemoembolization at our institution underwent CT scanning before each chemoembolization session.
The respective scanning parameters used for the 4-, 8-, 16- and 64-multidetector row CT scanners included the following: (detector configurations) 4 × 2.5, 8 × 1.25, 16 × 0.75, and 64 × 0.625 mm; (slice thickness) 3.2, 2.5, 3, and 3 mm; (reconstruction intervals) 3, 2.5, 3, and 2 mm; (table speed) 12.5, 13.5, 24 and 46 mm/rotation; (rotation time) 0.5-0.75 seconds.
Each CT examination included four phases that consisted of unenhanced images, hepatic arterial, portal venous, and equilibrium phases. After first acquiring unenhanced liver images in the craniocaudal direction, contrast medium (Iopromide, Ultravist 370; Schering, Berlin, Germany) was administered, which followed by a 30-mL sterile saline flush using a power injector (Multi-level CT; Medrad, Pittsburgh, PA). Contrast medium and saline solution were injected at 3 mL/sec through an 18-gauge plastic intravenous catheter placed in an antecubital vein. Contrast medium volume (delivered at 2 mL/kilogram body weight) varied from 100-130 mL. Hepatic arterial phase scan delays were 11-17 seconds after the descending aorta enhancement reached 100 Hounsfield units (HU), as measured using a bolus-tracking technique. Portal venous phase interscan delays were 20-30 seconds. The equilibrium phase commenced 180 seconds after completing the administration of contrast medium.
All angiographic examinations were performed by an experienced interventional radiologist (with 15 years of experience). Conventional chemoembolizations were performed as selectively as possible through the segmental or subsegmental arteries using a microcatheter (Microferret; Cook, Bloomington, IN or Progreat; Terumo, Tokyo, Japan). Initially, an iodized oil (Lipiodol; Andre Guerbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) emulsion was administered into the feeders. The iodized oil volume ranged from 3 mL to 10 mL, and the amount of doxorubicin ranged from 20 mg to 50 mg. Gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI) that were mixed with mitomycin (Mitomycin-10; Kyowa Hakko Kogyo, Tokyo, Japan) and contrast material were administered into the feeders until the blood flow had nearly completely stopped. Every effort was made to seek and catheterize small arterial feeders that supplied tumor thrombi. Additional superselective cisplatin (Cisplan; Donga, Seoul, Korea) infusion was started after obtaining near complete stasis of vascular flow of the target arteries. Cisplatin was prepared as a solution at a concentration of 0.5 mg/mL, and the drug was infused as selectively as possible at a rate of 5-10 mL/min. The total amount of cisplatin ranged from 70 mg to 100 mg according to the patient body weight.
All CT scans were reviewed, and four CT examinations in each patient were analyzed quantitatively, including the initial CT scan, the CT scan after the first chemoembolization, the CT scan after the last chemoembolization session, and the last CT scan during follow-up. CT scans were retrospectively reviewed by two radiologists and focused on the following parameters: the extent of portal vein thrombosis, the diameter of the affected portal vein (MPV, right portal vein [RPV] and left portal vein [LPV]), enhancement of portal vein thrombosis, and the existence of a collateral vein as depicted on CT scans. Enhancement of portal vein thrombosis was assessed by the use of a region of interest (ROI) measurement, which was an average value of three ROI values of 20 mm2 in the tumor thrombus. In the case of iodized oil uptake for portal vein thrombosis, an ROI measurement was not possible.
The mean follow up period was 34 months (range, 25-45 months; median, 33 months) after the initial CT scan, and 26 months (range, 16-40 months; median, 24 months) after the last chemoembolization session. The median number of chemoembolization procedures was 3 (range, 2-7), with a two- to four-month interval.
In all cases, the tumors were massive HCCs with MPV thrombosis as depicted on CT images. The primary tumors were in the right lobe of the liver (n = 4), in the caudate lobe (n = 1), and in the portal vein itself (n =2). The extent of portal vein thrombosis at the initial presentation was variable; LPV and MPV (n = 1), RPV and MPV (n = 3), as well as RPV, LPV, and MPV (n = 3). The involved portal vein was the LPV (n = 4), RPV (n = 6), right anterior portal vein (RAPV) (n = 6), right posterior portal vein (RPPV) (n = 6), and MPV (n = 7). The mean diameters of the affected MPV, RPV and LPV were 17.3, 19.2 and 14.6 mm, respectively. The mean attenuation of the portal vein tumor thrombosis as depicted on initial CT images was as follows: 45 HU for the precontrast phase, 81.5 HU for the arterial phase, and 84 HU for the portal phase. A collateral vein around the gall bladder was noted at the initial presentation in the four patients.
Based on follow-up studies, the extent and diameter of the affected portal vein decreased (Fig. 1). Portal vein thrombosis was present in the LPV (n = 2), RPV (n = 2), RAPV (n = 5), RPPV (n = 3), and MPV (n = 2) as depicted on the CT images obtained after the last chemoembolization session. Portal vein thrombosis was present in the LPV (n = 1), RPV (n = 2), RAPV (n = 5), RPPV (n = 3), and MPV (n = 1), as depicted on the last CT images obtained during the follow-up examination (Table 1). Whereas portal vein thrombosis was completely resolved in only one patient, a residual thrombus without viability was persistent in six patients. In three patients, segmental obliteration of the LPV (n = 1), RPV (n = 1), and RAPV (n = 1) was noted. The mean diameters of the MPV, RPV, and LPV were 15.4, 15.5 and 12.2 mm after the first chemoembolization session and 14.1, 9.2, and 7.5 mm after the last chemoembolization session and 12.8, 8.4, and 7.3 mm as measured at the last follow-up, respectively (Table 2).
In three patients, the ROI measurement of the portal vein thrombosis was not possible because of iodized oil retention. In the four remaining patients, the mean attenuation of portal vein tumor thrombosis after the last chemoembolization session was 37 HU for the precontrast phase, 46.5 HU for the arterial phase, and 50 for the portal phase.
A collateral vein was noted in five patients after the first session of chemoembolization, whereas a collateral vein was present in all patients after the last session of chemoembolization. In two patients, a collateral vein was present only around the gall bladder. In five patients, a collateral vein was present around the LPV (n = 1), PRV (n = 2), as well as the MPV, RPV and LPV (n = 2).
The mean α-fetoprotein level was measured to be 7,512 ng/mL (range, 34-34,100 ng/mL) at initial presentation. In six patients, the baseline α-fetoprotein level was higher than 300 ng/mL, which represents a decrease of more than 50% after the first chemoembolization session. In all patients, the α-fetoprotein level was less than 20 ng/mL than following the last chemoembolization session.
The diagnosis of malignant portal vein thrombosis (PVT) has been based on characteristic CT findings including an expansile vein diameter and relatively strong enhancement or obvious direct invasion by a tumor in the adjacent liver parenchyma (12-14). In our study, a malignant PVT was seen with a decreased portal vein diameter and enhancement of the thrombi after chemoembolization, but residual thrombi without viability can be persistent for months or years.
After repeated chemoembolization, the mean diameter of the affected portal vein significantly decreased. Especially the RPV, which was involved to the greatest degree, demonstrated a marked decrease in the diameter as seen on follow-up CT images. The extent of portal vein thrombosis decreased after repeated chemoembolization, if chemoembolization was found to be effective. However, the complete resolution of portal vein thrombosis was observed in only one patient. In six patients, various degrees of residual thrombosis were present from one to three years after the last treatment. Obvious enhancement of PVT, as seen on arterial phase images, indicates viable tumor thrombosis. However, in three patients, it was not possible to measure the enhancement of tumor thrombi on follow-up CT scans after repeated chemoembolization due to retention of iodized oil in the thrombi. In four patients, residual thrombi without viability showed little enhancement, as seen on contrast-enhanced CT scans. Thus, portal vein thrombosis depicted on a follow-up CT scan should not be diagnosed as a residual viable tumor if the portal vein thrombosis shows little enhancement and the extent of thrombosis does not increase.
Spiral CT performed at least four weeks after treatment is currently accepted as the standard imaging modality for the assessment of response to treatment for HCCs (15). Non-enhanced tumoral areas reflect tissue necrosis after treatment, whereas viable neoplastic cells are recognized by enhanced areas inside the treated lesions (15). Whereas the use of iodized oil may diminish the reliability of the CT scans, the accuracy of MRIs is not affected (15). Thus, a further investigation of the usefulness of MRI for the assessment of PVT may be needed.
Some investigators have noted that chemoembolization was contraindicated for patients with MPV tumor thrombosis, because of the potential risk of ischemic liver damage and no survival benefit (8). In contrast, other authors insisted that chemoembolization might be safely performed in patients with MPV tumor thrombosis if they have good hepatic reserve and collateral circulation around the portal vein (5-7). Chung et al. (6) performed a chemoembolization on 110 patients with HCC that invaded the 1st branch of the portal vein or the MPV: The response rate was 28% and the median survival time was 6 months. In their series, three patients died within one month after chemoembolization and tumor extent was a significant factor in predicting the efficacy of therapy. They insisted that chemoembolization was effective and safe if the extent of the tumor was limited and liver function was preserved. Georgiades et al. (5) reported that the mortality rate within one month was zero in HCC patients with PV thrombosis, whereas the Child-Pugh score was the strongest prognostic factor. At our hospital, whereas chemoembolization is not attempted in the patients with MPV tumor thrombosis if the patients are classified into Child-Pugh C class, chemoembolization is strongly recommended in patients with Child-Pugh A and B class.
Prominent collateral vessels are known to have protective properties against acute hepatic failure following chemoembolization in HCC with MPV tumor thrombus (7). In our study, however, four patients had collateral veins around the gall bladder and no patients had collateral veins around the MPV or first branch of the portal vein at the initial presentation. Since this study included only well-controlled patients with MPV tumor thrombosis by chemoembolization, a paucity of collateral veins in our study population does not guarantee that chemoembolization is safe in HCC patients with MPV thrombosis without collateral veins.
Our study had several limitations; first, the tumor thrombi were not confirmed histopathologically. Second, only a small number of patients were involved in the study. Third, we used CT scanning for the evaluation of residual viable tumors and thus it was not possible to measure the enhancement of tumor thrombi on follow-up CT scans after repeated chemoembolizations in three patients.
In conclusion, if chemoembolization is effective in patients with an HCC that invades the portal vein, the extent and enhancement of PVT is reduced, but residual thrombosis frequently persists months or years without evidence of a viable tumor.
Figures and Tables
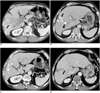 | Fig. 1Imaging findings are presented for 64-year-old man with hepatocellular carcinoma accompanied by main portal vein invasion.
A. CT scan of portal venous phase obtained at initial presentation shows ill-defined tumor (arrows) in liver parenchyma and thrombus (arrowhead) in main portal vein. Expansion of portal vein and enhancement of thrombus are evident.
B. One month after initial chemoembolization, tumor (arrow) was spotted with iodized oil in liver parenchyma and tumor thrombus (arrowhead) laden along with iodized oil in right anterior portal vein on portal phase.
C. Also noted is decreased extent and diameter of thrombus (arrowhead) in portal vein on same CT scan as B.
D. CT scan of portal venous phase obtained 34 months after last chemoembolization shows small thrombus (arrow) in right anterior portal vein.
|
References
1. El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008. 134:1752–1763.
2. Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009. 10:425–434.
3. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003. 37:429–442.
4. Myung SJ, Yoon JH, Gwak GY, Shin CM, Ahn DW, Yu SJ, et al. A case of infiltrative hepatocellular carcinoma with main portal vein tumor thrombosis successfully treated by transarterial chemoembolization. Korean J Hepatol. 2006. 12:107–111. [Korean].
5. Georgiades CS, Hong K, D'Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005. 16:1653–1659.
6. Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995. 165:315–321.
7. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997. 79:2087–2094.
8. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983. 148:397–401.
9. Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, et al. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006. 106:1990–1997.
10. Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005. 103:2419–2426.
11. Jeon UB, Chung JW, Cho YK, Park JH, Jae HJ, So YH. Superselective cisplatin infusion after conventional transarterial chemoembolization for the patients with hepatocellular carcinoma invading main portal vein. 2007. In : The Radiological Society of North America 93th Scientific Assembly and Annual Meeting;
12. Mathieu D, Grenier P, Lardé D, Vasile N. Portal vein involvement in hepatocellular carcinoma: dynamic CT features. Radiology. 1984. 152:127–132.
13. Shah ZK, McKernan MG, Hahn PF, Sahani DV. Enhancing and expansile portal vein thrombosis: value in the diagnosis of hepatocellular carcinoma in patients with multiple hepatic lesions. AJR Am J Roentgenol. 2007. 188:1320–1323.
14. Tublin ME, Dodd GD 3rd, Baron RL. Benign and malignant portal vein thrombosis: differentiation by CT characteristics. AJR Am J Roentgenol. 1997. 168:719–723.
15. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. European Association for the Study of the Liver. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001. 35:421–443.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download