Abstract
Objective
We wanted to compare the efficacy of the new CT response evaluation criteria for predicting the tumor progression-free survival (PFS) with that of RECIST 1.1 in non-small cell lung cancer (NSCLC) patients who were treated with bevacizumab.
Materials and Methods
Sixteen patients (M:F = 11:5; median age, 57 years) treated with bevacizumab and combined cytotoxic chemotherapeutic agents were selected for a retrospective analysis. The tumor response was assessed by four different methods, namely, by using RECIST 1.1 (RECIST), RECIST but measuring only the solid component of tumor (RECISTsolid), the alternative method reflecting tumor cavitation (the alternative method) and the combined criteria (the combined criteria) that evaluated both the changes of tumor size and attenuation. To evaluate the capabilities of the different measurement methods to predict the patient prognosis, the PFS were compared, using the log rank test, among the responder groups (complete response [CR], partial response [PR], stable disease [SD] and progressive disease [PD]) in terms of the four different methods.
Results
The overall (CR, PR or SD) response rates according to RECIST, RECISTsolid, the alternative method and the combined criteria were 81%, 88%, 81% and 85%, respectively. The confirmed response rates (CR or PR) were 19%, 19%, 50% and 54%, respectively. Although statistically not significant, the alternative method showed the biggest difference for predicting PFS among the three response groups (PR, SD and PD) (p = 0.07). RECIST and the alternative method showed a significant difference for predicting the prognosis between the good (PR or SD) and poor overall responders (p = 0.02).
Conclusion
The response outcome evaluations using the three different CT response criteria that reflect tumor cavitation, the ground-glass opacity component and the attenuation changes in NSCLC patients treated with bevacizumab showed different results from that with using the traditional RECIST method.
Accurately assessing the changes of the tumor burden is important when conducting cancer therapy, and so much effort has been directed toward the development of standardized and reproducible methods for evaluating the response of tumors. The Initial Response Evaluation Criteria in Solid Tumors (RECIST) published in 2000 (1) has been widely used in the scientific oncology community. The revised RECIST guidelines (version 1.1) were developed for current use (2). However, a number of questions and issues (e.g., tumor size changes only, not considering various morphologic changes within the target lesions with treatment) have been raised about the use of the traditional tumor response criteria, including the revised version 1.1. Particularly, the concern over the applicability of the RECIST criteria has been focused on the trials of molecularly targeted non-cytotoxic drugs, including angiogenesis inhibitors (2-5).
There are diverse tumor response patterns in non-small cell lung cancer (NSCLC) that is treated with vascular endothelial growth factor (VEGF) receptor inhibitor and platinum-based chemotherapy. Crabb et al. (3) observed cases that showed the appearance of cavitation and this implied there was a tumor response during the treatment of NSCLC with angiogenesis inhibitor plus chemotherapeutic agents. They proposed that the response assessment might be improved by incorporating cavitation into the volume assessment of target lesions, which potentially alters the outcome of key efficacy parameters in clinical trials. In another study on gastrointestinal stromal tumors (GISTs) that were treated with imatinib (4), dramatic changes were noted in the tumor attenuation values (due to tumor necrosis) after the treatment, as determined by measuring the CT attenuation coefficient (Hounsfield unit [HU]) on the contrast enhanced CT images. The authors of that study recommended incorporating the tumor attenuation changes in addition to the tumor size changes for tumor response evaluation.
In addition to size changes, cavitation and attenuation changes within a tumor (due to tumor necrosis and hemorrhage) are also tumor events in response to anticancer chemotherapy, and particularly to molecularly targeted non-cytotoxic drugs. The idea of diverse tumor responses to anticancer therapy prompted us to devise more appropriate CT response criteria than the conventional criteria to enhance predicting the disease progression free survival (PFS) in patients with NSCLC. Thus, the purpose of our study was, as a pilot study, to compare the efficacy of the CT response evaluation criteria (reflecting tumor cavitation, a ground-glass opacity [GGO] component and attenuation changes) for the prediction of tumor PFS with that of the RECIST 1.1 for NSCLC patients treated with bevacizumab (an anti-VEGF antibody).
Our Institutional Review Board approved our retrospective study with a waiver of informed consent; written informed consent was acquired for the use of CT scans for all the patients.
We acquired the patients' data from the prospective Phase II clinical trial of Bevacizumab as a salvage treatment for NSCLC, which was conducted at our institution during the period from May 2006 to July 2009. A total of 21 patients with metastatic or recurrent NSCLC and who were treated with bevacizumab (at a dose of 15 mg/kg on day 1) combined with cytotoxic chemotherapeutic agents (gemcitabine 1000 mg/m2 IV on days 1 and 8, and cisplatin 70 mg/m2 IV on day 1), were selected for this study. We excluded those patients (n = 2) who had not undergone post-treatment CT at our institution, or those (n = 3) who had no measurable disease on the pre-treatment CT. Ultimately, 16 patients (M:F = 11:5; median age, 57 years) were enrolled in this study. The median follow-up time was 7.9 months (range, 0.9-32.6 months).
The initial CT scans were performed within two weeks before starting chemotherapy, and the follow-up CT scans were conducted after two cycles of chemotherapy. For all the patients, the CT studies were obtained with a 64-detector (LightSpeed VCT XT, GE Healthcare, WI) row scanner and using the helical technique (125 mA, 120 kVp, a beam width of 10-20 mm, a beam pitch of 1.375-1.5). The scans covered from the lung apices to the level of the middle portion of both kidneys. Scanning was started 90 seconds after the intravenous injection of contrast medium (a total of 80 mL of Iomeron 300 [Iomeprol]; Bracco; Milan, Italy). The contrast medium was infused at a rate of 3 mL/s and by using a power injector (MCT Plus; Medrad; Pittsburgh, PA). The image data was reconstructed with a 2.5-mm section thickness. For the mediastinal window images, the image data was reconstructed with a soft-tissue algorithm, and for lung window images, the data was reconstructed with a bone algorithm. The reconstructed images were directly interfaced with a picture archiving and communication systems (PACS) (PathSpeed or Centricity 2.0; GE Healthcare, Mt. Prospect, IL), which displayed all the image data on two monitors (a 1536 × 2048 matrix, an 8-bit viewable gray scale and 60-ft-Lambert luminescence). Both the mediastinal (width: 400 HU, level: 20 HU) and lung (width: 1500 HU, level: -700 HU) window images were viewed on these monitors.
Two chest radiologists (both with five years of CT interpretation experience) and who were blinded to the clinical details and outcomes independently evaluated the CT images. The size of the target lesion was measured on the initial and follow-up CT scans at the level of the lesion equator (the longest diameter on the largest target lesion image plane). They recorded the changes in the size of the target lesion before and after the therapy once with including both the solid and GGO components (on lung window images) of the lesion ([RECIST guidelines version 1.1], designated as RECIST) and then at the other time with including only the solid component on the mediastinal window images of the lesion (designated as RECISTsolid) (6) (Fig. 1). Each target lesion was classified as being either cavitated or not cavitated. In a cavitary lesion, only the soft-tissue wall thickness, except the cavitary area, was measured as the tumor size (designated as the alternative measurement), that is, we subtracted the cavity diameter (zero if no cavity was present) from the longest diameter of the target lesion (3) (Fig. 2). Each measurement was performed on the same image plane. As for the attenuation measurements, the mean CT attenuation value of each target lesion was measured in HUs by drawing a region of interest covering as large an area as possible (at least two thirds of the longest diameter) in the solid portion of the target lesion. Necrotic areas were also included in the attenuation value measurements. The attenuation measurements were performed for the evaluation of the percentage changes in the mean lesion attenuation or the contrast enhancement changes before and after treatment (designated as the combined criteria for evaluating both the changes of the tumor size and attenuation based on Choi's criteria [4]) (Table 1). If a lesion had a small solid portion, then a region of interest of a 5 mm2 area was not applicable to measure the attenuation (e.g., a very thin cavitary wall in a cavitary lesion or a very small tumor after the treatment), and we were not able to measure the attenuation values of such a lesion. In these conditions, size measurements only were considered in the tumor response assessment. All the other details regarding the definitions for the designation of response and the assessment of non-target lesions for the three different assessment methods were identical to the RECIST version 1.1.
First, response assessment was performed by using the RECIST version 1.1 (2). Second, the tumor response was evaluated according to RECIST with covering only the solid component of the target lesions (RECISTsolid). Third, an alternative response assessment (the alternative method) was used to measure the tumor response and particularly in the cavitary lesions; we calculate the changes of the target lesion with including only the soft-tissue wall thickness component and we excluded the air component of cavitary change (subtracting the cavity diameter from the longest diameter of the cancer mass) (3). Finally, the combined criteria (Table 1) were applied for tumor response evaluation (4), for which both the size and attenuation changes on the enhanced scans before and after treatment were computed for each target lesion. Following independent interpretations by the two readers, discordant reports were jointly reviewed by the two readers and one third reader (with seven years of CT interpretation experience), and a final consensus decision was reached.
After treatment, the patients were followed up at two month intervals with chest and abdominal CT scans to identify any disease progression. The patients were also observed for survival until death or until the last contact if still alive. The patients were classified as patients with a complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) after treatment. The good responders were deemed as the patients with a CR or PR, and the good overall responders were deemed as the patients showing a CR, PR or SD on the follow-up CT.
Statistical analyses were performed with SPSS software (SPSS for Windows, version 15.0, 2006; SPSS, Chicago, IL), and significance was set at p values < 0.05.
Disease PFS was defined as the time from the starting date of treatment until the time point of patient progression (as shown by radiologic [CT] and clinical examination) or death from the disease. For the patients who had no evidence of progressive disease, the absence of disease progression was ascertained at the date of the last follow-up examination. We determined how much the response assessment would be altered if we applied the three different assessment evaluation methods instead of the RECIST method. To evaluate the capability of RECIST, RECISTsolid, the alternative method and the combined criteria to predict the patient prognosis, the PFSs were compared between the responders (CR, PR, SD and PD) or between the good and poor responders or between the good and poor overall responders according to the four different measurement methods. The comparisons were conducted using a log-rank test. The interobserver agreement between the two radiologists was assessed by calculating the k value for response according to each response criterion.
The details of the response designations by using the four different assessment methods for each patient are summarized in Table 2. Five patients in the trial of bevacizumab-containing chemotherapy were found to have cavitation within lesions after treatment. Marked cavitation (to the degree that there was no residual solid component) within the lesions occurred in two patients (Fig. 2).
Of the 16 patients, six RECIST poor responders would have achieved a designation of PR by the combined criteria. In the case of the alternative method, of the same six patients, five patients would have been reclassified as PR and one as SD. The overall response rates (ORRs, the patients showing a CR, PR or SD) according to RECIST, RECISTsolid, the alternative method and the combined criteria were 81%, 88%, 81%, and 81%, respectively. The confirmed response rates (RRs, the patients showing a CR or PR with the condition of response confirmation) were 19%, 19%, 50% and 56%, respectively.
Although no significant difference was observed for the PFS among the three (PR, SD and PD) response groups to vascular inhibitor therapy, the alternative method showed the biggest stratifying power among the three groups for the prediction of PFS (p = 0.07) (Fig. 3). For the prognosis between the good (CR or PR) and poor responders, the alternative method again showed the biggest stratifying power among the three groups for the prediction of PFS (p = 0.09), yet there is no statistically significance (Fig. 4). The RECIST and the alternative method showed a significant difference for the prognosis between the good (CR, PR, or SD) and poor overall responders (p = 0.02) (Fig. 5).
The interobserver agreements between the two radiologists for the RECIST and the newly devised criteria that reflected morphologic changes were all good (Table 3). The interobserver agreement for the alternative method was the best (κ = 0.79, 95% CI: 0.52-1.00), whereas that for the combined criteria was the worst (κ = 0.68, 95% CI: 0.36-0.99).
Evaluating a PR on an imaging study of solid tumors is not easy (7-9). Molecular-targeted treatment has given rise to a different antitumor effect from that of cytotoxic therapy and so this may highlight the limitations of using the size criteria alone for the assessment of a tumor response to the target agent (5). Furthermore, the response to bevacizumab, which has an antiangiogenic mechanism of action, may be inadequately assessed by the traditional size-based radiologic criteria (RECIST 1.0 or RECIST 1.1), which were designed for assessing the reduction of the tumor volume following the administration of cytotoxic agents. We present here three novel CT criteria that reflect the morphologic findings for predicting the response to bevacizumab-containing chemotherapy in patients with NSCLC. These criteria were reproducible, as was shown by the good interobserver agreement for the assessment of tumor response between the two independent radiologists.
Cavitation within a tumor may be a kind of tumor response to cancer therapy, and especially to VEGF receptor inhibitor therapy. Thus, the response evaluation can be improved by incorporating cavitation into the volume assessment for target lesions and this may change important outcome-measurement parameters. In our study, five patients were found to have cavitation in lesions after treatment with bevacizumab therapy. In the case of Figure 2, the entire solid component was changed into an air-filled thin-walled cystic lesion. However, the tumor response was assessed as SD according to the traditional RECIST method. In contrast, this patient achieved a PR according to the alternative method.
The combined criteria reflect the change of tumor enhancement and this helped confirm that RECIST significantly underestimated the tumor response. The decreased attenuation values of the target lesions on CT after cancer therapy histopathologically correspond to an area of tumor necrosis or cystic degeneration. Comparing the results of Figures 4 and 5, the discrepancies among the response rates and overall response rates were mostly attributable to the different definition of PR between the traditional RECIST 1.1 and the combined criteria. In the combined criteria, the lower-level cut-off value (from 30% in RECIST 1.1 to 10% in the combined criteria) for a PR in terms of a decreased tumor size is applied. With the combined criteria, five SDs according to RECIST 1.1 could be re-categorized as PRs. These five patients were in the same overall good response group both according to the RECIST 1.1 and the combined criteria (Fig. 5), yet they were reclassified into the good response group according to the combined criteria from the poor response group according to the RECIST 1.1 (Fig. 4). This reclassification better reflects the recently changed concept of SD; achieving SD has been identified as a potential surrogate end point for an improved clinical outcome for epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) therapy. Currently, durable modest regression or prolonged SD achieved by these agents is viewed as evidence of antitumor activity (9). The recent studies that assessed tumors' responses to antiangiogenic agents with using CT or MRI have focused on tumor perfusion (10, 11). However, the degree of tumor enhancement has been consistently assessed because of variations in the scanning techniques. The Choi criteria can be simply and efficiently applied to response evaluation in daily practice, whereas the tumor perfusion evaluation method, despite that it is a sophisticated and accurate method, it requires repeated scans over time and this may impose a potential radiation hazard on patients who are undergoing CT perfusion evaluation (4).
The extent of ground-glass opacity in a peripheral lung cancer and especially in an adenocarcinoma (a partly solid nodule on CT scans) may be a prognosis determinant (6). However, the extent of GGO within a partly solid lung cancer does not vary much even after effective chemotherapy. Only the solid component within a partly solid nodule may show a response to cancer therapy, as is shown in Figure 1. Therefore, the measurement of changes in the solid component in the partly solid peripheral lung cancers may reflect accurately the tumor response to chemotherapy. Even though our results using the RECISTsolid method did not show a significant result for the prediction of PFS during bevacizumab therapy, this might have been caused by too small a number of patients who had a partly solid peripheral lung cancer.
When assessing a peripheral lung cancer response to anticancer therapy, RECIST 1.0 or RECIST 1.1 do not elaborate on the image viewing methods and specifically on whether we should use the lung or mediastinal window images of CT scans (1, 2). Thoracic radiologists conventionally measure the tumor size on the lung window images (including both the GGO and solid components in a partly solid tumor). Yet as mentioned above, only the solid component may show a response to anticancer therapy. Thus, the mediastinal window images may be more appropriate in the assessment of tumor response, and particularly in peripheral lung cancers (partly solid nodules).
Our study has some limitations. First, our study was a retrospective study (although the patients were enrolled in a prospective trial). Second, our results were based on a small number of cases. Thus, our results may not represent the overall results and they cannot be to freely generalized. Third, the applicability of these criteria should be tested with other biologic agents other than bevacizumab for treating NSCLC.
In conclusion, the response outcome evaluations using three different CT response criteria reflecting tumor cavitation, the GGO component and the attenuation changes in NSCLC patients treated with bevacizumab showed different results from that with using the traditional RECIST method. Our results indicate that assessing the morphologic characteristics, not to mention measuring the tumor size, may reflect the more detailed changes within tumors with this therapy and so give clinically useful response information as a noninvasive surrogate marker for predicting the prognosis. Further studies with a large number of NSCLC patients are needed for developing new response evaluation methods that combine and integrate the changes of the morphologic and size characteristics of target lesions.
Figures and Tables
Fig. 1
RECIST versus RECIST for measuring only solid portion (RECISTsolid).
A. Diagram depicting target lesion measurement by RECIST and RECISTsolid.
B, C. Solid component within partly solid nodule has disappeared after treatment, but ground-glass component of lesion is still remaining (patient No. 8).

Fig. 2
Response evaluation using alternative method.
A. Diagram depicting target lesion measurement by RECIST and alternative method.
B, C. CT images demonstrating pulmonary cavitation after two cycles of Bevacizumab-containing chemotherapy; CT images at baseline (B) and after two cycles of bevacizumab (C). There is no residual solid component within lesion. Tumor response was assessed as stable disease according to traditional RECIST. In contrast, it corresponds to partial response by alternative method (patient No. 9).
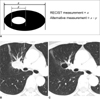
Fig. 3
Progression free survival in subgroups of partial response, stable disease and progressive disease according to each response criterion.
Although it was not statistically significant, prediction of response to vascular inhibitor therapy was better with using alternative method (p = 0.07) than with using RECIST, RECISTsoild or combined criteria.
PD = progressive disease, PFS = progression-free survival, PR = partial response, SD = stable disease

Fig. 4
Progression free survival in good and poor responders by each response criterion.
Although it was not statistically significant, prediction of response to vascular inhibitor therapy was better with using alternative method (p = 0.09) than with using RECIST, RECISTsoild or combined criteria. PFS = progression-free survival

Fig. 5
Progression free survival in good and poor overall responders by each response criterion.
RECIST and alternative method show significant difference for predicting prognosis between good and poor overall responders (p = 0.02). PFS = progression-free survival

Table 1
Tumor Response According to Choi Criteria

Note.-* Sum of longest diameters of target lesions as defined in RECIST (Reference 2)
References
1. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
2. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.
3. Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009. 27:404–410.
4. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007. 25:1753–1759.
5. Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, et al. High-grade soft-tissue sarcomas: tumor response assessment--pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009. 251:447–456.
6. Lee HY, Han J, Lee KS, Koo JH, Jeong SY, Kim BT, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer. 2009. 66:379–385.
7. Curran SD, Muellner AU, Schwartz LH. Imaging response assessment in oncology. Cancer Imaging. 2006. 6:S126–S130.
8. Gwyther SJ. Current standards for response evaluation by imaging techniques. Eur J Nucl Med Mol Imaging. 2006. 33:11–15.
9. Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004. 22:4442–4445.
10. Sabir A, Schor-Bardach R, Wilcox CJ, Rahmanuddin S, Atkins MB, Kruskal JB, et al. Perfusion MDCT enables early detection of therapeutic response to antiangiogenic therapy. AJR Am J Roentgenol. 2008. 191:133–139.
11. Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY, et al. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology. 2005. 237:151–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download