Abstract
Objective
To describe the radiographic findings of primary pulmonary tuberculosis (TB) in previously healthy adolescent patients.
Materials and Methods
The Institutional Review Board approved this retrospective study, with a waiver of informed consent from the patients. TB outbreaks occurred in 15 senior high schools and chest radiographs from 58 students with identical strains of TB were analyzed by restriction fragment length polymorphism analysis by two independent observers. Lesions of nodule(s), consolidation, or cavitation in the upper lung zones were classified as typical TB. Mediastinal lymph node enlargement; lesions of nodule(s), consolidation, or cavitation in lower lung zones; or pleural effusion were classified as atypical TB. Inter-observer agreement for the presence of each radiographic finding was examined by kappa statistics.
Results
Of 58 patients, three (5%) had normal chest radiographs. Cavitary lesions were present in 25 (45%) of 55 students. Lesions with upper lung zone predominance were observed in 27 (49%) patients, whereas lower lung zone predominance was noted in 18 (33%) patients. The remaining 10 (18%) patients had lesions in both upper and lower lung zones. Pleural effusion was not observed in any patient, nor was the mediastinal lymph node enlargement. Hilar lymph node enlargement was seen in only one (2%) patient. Overall, 37 (67%) students had the typical form of TB, whereas 18 (33%) had TB lesions of the atypical form.
Pulmonary tuberculosis (TB) has been classified into primary and reactivation (post-primary) forms (1). In about 5% of individuals infected by Mycobacterium tuberculosis (M. tuberculosis), the infection progresses to active disease within two years after infection. This progressive primary TB is considered to occur typically in childhood. An additional 5% develop active disease at some later point in their lives, and this reactivation TB is considered to occur typically in adults (2).
Traditionally, it has been thought that the radiographic manifestations of primary TB infection are distinct from those of reactivation TB (1). Mediastinal lymph node enlargement, lower lobe lesions, and pleural effusions are considered to be characteristics of primary TB infection, whereas upper lobe lesions, cavitation, and fibrosis are considered to be typical of reactivation TB (3-5). However, recent studies using genotyping methods for M. tuberculosis isolates have shown that the radiographic features are often similar in patients who apparently have primary disease by recent infection and those who have reactivation TB by remote infection (6, 7).
To confirm that TB in an adult is due to recent infection, we document recent tuberculin skin test conversions or utilize restriction fragment length polymorphism (RFLP) analysis (DNA fingerprinting with the IS6110 insertion sequence) of M. tuberculosis isolates (8-10). Isolates from patients infected with epidemiologically unrelated strains of TB have different RFLP patterns, whereas those from patients with epidemiologically linked strains generally have identical RFLP patterns. Therefore, clustered cases of TB, defined as those in which the isolates have identical or closely related genotypes, have usually recently been transmitted. To evaluate the radiographic findings of primary pulmonary TB in previously healthy adolescents, we reviewed the chest radiographs of a large number of patients with TB whose isolates had been subjected to RFLP analysis.
The Institutional Review Board approved this retrospective study, with a waiver of informed consent from the patients.
From January 2007 to December 2009, TB outbreaks occurred in 15 senior high schools in South Korea. By reviewing the medical records of the Korean Institute of Tuberculosis, we identified all 90 students in whom culture-proved TB demonstrated identical strains of TB by RFLP analysis with the IS6110 insertion sequence. All isolates from the same school appeared to be the same M. tuberculosis strain.
Ministry of Education, Science and Technology of Korea performs student medical check-ups when students are in the first and fourth grades of elementary school and in the first grade of middle and high school. The students' medical check-up includes chest radiographic examination for the evaluation of pulmonary TB. All 90 students in our study also underwent chest radiographic examination in the first grade of middle or high school. Because all these students were previously healthy and had normal chest radiographs in their previous student medical check-ups, we considered this recent infection proven by RFLP analysis as primary TB. The mean interval between the time of the last normal chest radiographs and the time of TB diagnosis for each patient was 1.25 years (range; 0.5-2.5 years). The mean age of these 90 students was 17 ± 1.2 years, and 64 students (71%) were male. Underlying chronic disease was not reported in any student. Moreover, none of the students with active pulmonary TB had a previous history of TB treatment.
All these students were referred to public health centers, where they received formal chest radiographic examinations with a regular-sized (14 × 17-inch) film (n = 32) or digital radiographs (n = 58). Of the 90 students, initial chest radiographs were available in 58 students who underwent chest radiographic examinations with a digital radiographic (radiographic units from various vendor companies) technique. Imaging parameters for digital radiography were as follows: image size, 14 × 17-inch or 17 × 17-inch; maximum tube currents, 650 mA; usual exposure amount, 1 or 2 mAs; tube voltage, 100-120 kVp; focal spot size, 1.2 mm; detector-focus distance, 183 cm. Chest radiographic examinations were performed by the postero-anterior view only. Thus, these 58 students constituted the study population for the analysis of chest radiographic characteristics.
All chest radiographic image data of the 58 patients were directly interfaced to a picture archiving and communications system (M-view; Marotec Medical System, Seoul, Korea) which allowed to display all image data on monitors (four monitors, 2048 × 2560 image matrices, 10-bit viewable gray scale, and 145.9-ft-lambert luminescence).
The initial chest radiographs of the students with newly diagnosed TB were reviewed independently by two chest radiologists who had 21 and eight years of experience, in chest radiology respectively, and differences in observed findings were resolved by consensus. The observers assessed the presence of lung parenchymal abnormalities including nodule(s), consolidation, and cavities. The presence or absence of pleural effusion and lymph node enlargement of the mediastinum or hilum was also recorded. Nodule(s) (≤ 3 cm in diameter; large nodules, ≥ 10 mm in diameter, small nodules, < 10 mm in diameter) were considered present when there was a rounded opacity, either well or poorly defined. Consolidation was defined as a homogeneous increase in pulmonary parenchymal opacity that obscured the margins of vessels and airway walls. A cavity was diagnosed when an air-filled space was noticed within the pulmonary consolidation, mass, or nodule (11).
The distributions (upper or lower zone) and the laterality (unilateral or bilateral) of lung lesions were also analyzed. Lesions were considered to be in the upper lung zone if cephalad to the pulmonary hila and in the lower lung zone if caudad to the hila.
After the analysis of chest radiographic findings, the findings were considered typical of the previous definition of reactivation pulmonary TB by remote infection if lesions of consolidation, nodule(s), or cavities were present in the upper lung zone(s). The presence of concurrent hilar lymphadenopathy, a lower lung lesion, or pleural effusion did not change the characterization of typical TB. The findings were regarded to be atypical if mediastinal lymph node enlargement, lower lung zone abnormalities, or a pleural effusion was present. Radiographs with a cavitary lesion or segmental or lobar consolidation in the lower lung zones were also considered atypical (5-7).
Statistical analyses were performed using commercially available software (SPSS 15.0; SPSS, Chicago, IL). The agreement between the two radiologists for the presence or absence of each radiographic finding was examined by using the k statistic. A k-value of 0-0.20 indicates slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement; and 0.81-1.00, almost perfect agreement.
Two observers had almost perfect agreement for the identification of mediastinal lymph node enlargement (k = 1.00), hilar lymph node enlargement (k = 1.00), pleural effusion (k = 1.00), large nodule (k = 0.965), cavity (k = 0.894), and consolidation (k = 0.813). There was substantial agreement between the two radiologists for the identification of small nodules (k = 0.742).
Of the 58 patients that underwent chest radiographs, three had normal chest radiographs. Table 1 demonstrates summarized abnormal chest radiographic findings in remaining 55 patients. Cavitary lesions were present in 25 (45%) students. Pleural effusion was not observed in any patient, nor was mediastinal lymph node enlargement. Hilar lymph node enlargement was seen in only one patient (2%).
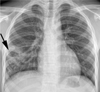
Lesions with upper lung zone predominance were observed in 27 (49%) patients and lesions with lower lung zone predominance were observed in 18 (33%) patients. Remaining 10 (18%) patients had lesions in both upper and lower lung zones. Bilateral involvement of lung lesions was observed in 13 (24%) patients. Overall, 37 (67%) students had the typical form of reactivation TB (Figs. 1, 2), and 18 (33%) had TB lesions of the atypical form, based on chest radiograph findings (Fig. 3).
The aim of this study was to describe the radiographic findings of primary pulmonary TB in previously healthy adolescent patients with recent infection. We found that primary pulmonary TB in our teenage high-school students typically present with upper lobe nodule(s), consolidation, or cavitary lesion(s) on chest radiographs. Mediastinal lymph node enlargement or pleural effusion was not seen in our patients. These findings have been traditionally considered as typical chest radiographic findings of reactivation TB with remote infection. In reactivation TB, the chest radiographs have been regarded to show patchy consolidation and poorly-defined nodules involving the upper lobes. In one-third of patients, cavities are present within lung abnormalities (12, 13).
Primary TB has been considered to be mainly a disease of infancy and childhood. The most common radiographic abnormalities of primary TB in infancy and childhood are intra-thoracic lymph node enlargement, pleural effusion, and lower lobe lung lesions (14-17). Primary TB can also occur in adults and hence a shift toward delayed presentation in adults may be related to a decrease in childhood exposure and an increasing number of immunocompromised hosts (14). Primary tuberculosis in adolescents and adults tends to manifest itself as lung parenchymal lesions in the upper lobes or superior segments of the lower lobes (14, 17). In addition, pleural effusion or mediastinal lymph node enlargement is occasional. Cavitation, usually within area of consolidation, can also occur in adolescent or adult primary TB as in our cases. Early cavitation in primary TB is more common and occurs more quickly in adults than in any other age group (14). Therefore, primary TB in adolescents and adults can manifest upper lobe cavitary consolidation without mediastinal or hilar lymph node enlargement or pleural effusion, and thus show traditionally-regarded typical chest radiographic findings of reactivation TB with remote infection.
The radiographic findings observed in our study concur with those examined in the study of Sant'Anna et al. (18), who evaluated radiographic findings of pulmonary TB observed in the adolescent age group. In their study, although mode (primary, endogenous reactivation or exogenous reinfection) of infection was not clearly mentioned, lung parenchymal lesions were located in the upper lobes in 57% of patients, whereas cavitary lesions occurred in 183 (32%) of 564 patients (28% [67 of 243 patients] consisting of 10 to15 year old adolescents and 36% [116 of 321] consisting of 16 to 19 adolescents) (18).
Recent studies based on DNA fingerprinting suggest that chest radiographic features are similar in patients who apparently have primary disease and those who have reactivation TB (6, 7). Additionally, more than 70% of adult patients with TB pleurisy (which had been regarded as a primary TB manifestation rather than reactivation TB) had features of reactivation TB in the lung parenchyma (19). Moreover, cavitary lung lesions do occur within six months of initial infection; in other words, cavitary lesions manifest as radiographic findings of primary TB pulmonary infection (20). These observations suggest that typical reactivation-type pulmonary TB can result from primary infection, endogenous reactivation, or exogenous reinfection (21, 22).
Impaired host immunity has been regarded as a predisposing factor for TB disease. Human immunodeficiency virus (HIV)-seropositive pulmonary TB patients with crucial immunodeficiency (CD4 T lymphocyte count, < 200/mm3) have a higher prevalence of mediastinal lymphadenopathy and a lower prevalence of cavitation than do HIV-seronegative patients (23, 24). Previous studies demonstrated that these radiologic findings of TB in HIV-infected patients reflect impaired cell-mediated immunity (6, 7). Thus, the traditional concept of differences in chest radiographic findings between children and adults with TB disease may reflect differential efficacy of the immune response, rather than differences in the timing of infection (6, 7). An important predictor of radiographic appearance may be the integrity of the host immune response, as determined by patient age and immunodeficiency (25). Neonate, young children, or HIV-infected persons who have impaired cell-mediated immune responses show a tendency to have the atypical form of TB, whereas immunocompetent patients tend to have the typical form of previously known reactivation reactivation TB (6, 7).
Several characteristics of our study population were unique; all were previously healthy senior high school students, with a mean age of 17 years, and no patient had any underlying chronic illness. All students were demonstrated to be infected with an identical strain of M. tuberculosis at each school, which was proven by DNA fingerprint testing. These findings suggest that our adolescent patients were recently infected and they had recently developed primary pulmonary TB.
Our study has several limitations. First, our study subjects were senior high school students (adolescents). Thus, our results may not be generalized to children or adults. Second, chest radiographs of all patients were not available; thus, a selection bias may be present. Third, we evaluated radiographic findings only, even in the posteroanterior direction only; thus, we might not have found mediastinal or hilar lymph node enlargement or minimal pleural effusion. In addition, three students in our study had normal chest radiographs, despite having culture-confirmed active TB. It has been reported that the radiographs may be normal or show only mild or nonspecific findings in patients with active disease (12). Common causes of a missed diagnosis of TB are failure to recognize hilar and mediastinal lymphadenopathy and the oversight of mild parenchymal abnormalities such as small centrilobular nodules. However, inter-observer agreement in the identification of hilar or mediastinal lymph node enlargement and pleural effusion were almost perfect in our study. Fourth, because we did not have enough data on serial tuberculin skin test results, students with previously normal chest radiographs and no history of tuberculosis were regarded to have primary TB infection. Thus, we used a broad definition of primary TB infection (14). Finally, we did not evaluate the effect of BCG vaccination on the host immune response and radiologic manifestation of TB infection. Our national policy for preventing tuberculosis recommends BCG vaccination in the neonatal period. BCG vaccination may affect host immune response and radiologic manifestations of TB infection.
In conclusion, the most common radiographic findings of primary pulmonary TB by recent infection in previously healthy adolescents are upper lung lesions, including nodule(s), consolidation, and cavitation, which were previously thought to be typical radiographic findings of reactivation pulmonary TB by remote infection.
Figures and Tables
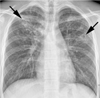 | Fig. 1Primary pulmonary tuberculosis in 18-year-old boy with typical radiographic findings. Chest radiograph shows patchy consolidation, nodules, and cavities (arrows) in bilateral upper lung zones. |
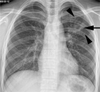 | Fig. 2Pulmonary tuberculosis in 18-year-old boy with typical radiographic findings. Chest radiograph shows cavitary nodule (arrow) with multiple small nodules (arrowheads) in left upper lung zone. |
References
1. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000. 161:1376–1395.
2. Small PM, Fujiwara PI. Management of tuberculosis in the United States. N Engl J Med. 2001. 345:189–200.
3. Lee KS, Song KS, Lim TH, Kim PN, Kim IY, Lee BH. Adult-onset pulmonary tuberculosis: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1993. 160:753–758.
4. Lee JY, Lee KS, Jung KJ, Han J, Kwon OJ, Kim J, et al. Pulmonary tuberculosis: CT and pathologic correlation. J Comput Assist Tomogr. 2000. 24:691–698.
5. Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008. 191:834–844.
6. Jones BE, Ryu R, Yang Z, Cave MD, Pogoda JM, Otaya M, et al. Chest radiographic findings in patients with tuberculosis with recent or remote infection. Am J Respir Crit Care Med. 1997. 156:1270–1273.
7. Geng E, Kreiswirth B, Burzynski J, Schluger NW. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA. 2005. 293:2740–2745.
8. Tabet SR, Goldbaum GM, Hooton TM, Eisenach KD, Cave MD, Nolan CM. Restriction fragment length polymorphism analysis detecting a community-based tuberculosis outbreak among persons infected with human immunodeficiency virus. J Infect Dis. 1994. 169:189–192.
9. Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994. 330:1703–1709.
10. Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, et al. Transmission of tuberculosis in New York City. An analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994. 330:1710–1716.
11. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008. 246:697–722.
12. Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol. 1986. 146:497–506.
13. Krysl J, Korzeniewska-Kosela M, Müller NL, FitzGerald JM. Radiologic features of pulmonary tuberculosis: an assessment of 188 cases. Can Assoc Radiol J. 1994. 45:101–107.
14. Choyke PL, Sostman HD, Curtis AM, Ravin CE, Chen JT, Godwin JD, et al. Adult-onset pulmonary tuberculosis. Radiology. 1983. 148:357–362.
15. Khan MA, Kovnat DM, Bachus B, Whitcomb ME, Brody JS, Snider GL. Clinical and roentgenographic spectrum of pulmonary tuberculosis in the adult. Am J Med. 1977. 62:31–38.
16. Kim WS, Choi JI, Cheon JE, Kim IO, Yeon KM, Lee HJ. Primary tuberculosis in infants: radiographic and CT findings. AJR Am J Roentgenol. 2006. 187:1024–1033.
17. Leung AN, Müller NL, Pineda PR, FitzGerald JM. Primary tuberculosis in childhood: radiographic manifestations. Radiology. 1992. 182:87–91.
18. Sant'Anna C, March MF, Barreto M, Pereira S, Schmidt C. Pulmonary tuberculosis in adolescents: radiographic features. Int J Tuberc Lung Dis. 2009. 13:1566–1568.
19. Kim HJ, Lee HJ, Kwon SY, Yoon HI, Chung HS, Lee CT, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest. 2006. 129:1253–1258.
20. Marais BJ, Parker SK, Verver S, van Rie A, Warren RM. Primary and postprimary or reactivation tuberculosis: time to revise confusing terminology. AJR Am J Roentgenol. 2009. 192:W198.
21. Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the prechemotherapy era. Int J Tuberc Lung Dis. 2004. 8:392–402.
22. Andronikou S, Vanhoenacker FM, De Backer AI. Advances in imaging chest tuberculosis: blurring of differences between children and adults. Clin Chest Med. 2009. 30:717–744.
23. Barnes PF, Bloch AB, Davidson PT, Snider DE Jr. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991. 324:1644–1650.
24. Leung AN, Brauner MW, Gamsu G, Mlika-Cabanne N, Ben Romdhane H, Carette MF, et al. Pulmonary tuberculosis: comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Radiology. 1996. 198:687–691.
25. Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008. 8:498–510.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download