Abstract
Objective
To evaluate the usage of duplex power Doppler ultrasound (PDUS) for the differentiation of benign and malignant thyroid nodules.
Materials and Methods
We prospectively examined 77 thyroid nodules in 60 patients undergoing ultrasound-guided fine needle aspiration biopsy (FNAB). Each nodule was described according to size, inner structure, borders, parenchymal echogenicity, peripheral halo formation, and the presence of calcification (B-mode ultrasound findings). Vascularity as determined by PDUS imaging was defined as non-vascular, peripheral, central, or of mixed type. For each nodule, the pulsatility index (PI) and resistive index (RI) values were obtained. Results of FNAB and surgical pathological examination (if available) were used as a proof of final diagnosis to categorize all nodules as benign or malignant. A receiver operating characteristic (ROC) curve analysis was performed to establish cut-off, sensitivity, and specificity values associated with RI-PI values.
Results
A significant relationship was observed between malignancy and irregular margins, microcalcifications, and hypoechogenicity on ultrasound examination (p < 0.05). The pattern of vascularity as determined by PDUS analysis was not a statistically significant criterion to suggest benign or malignant disease in this study (p > 0.05). The central, peripheral, and mean RI-PI values were higher in malignant nodules when compared to the other cytologies (p < 0.05).
Thyroid nodules are common, but an accurate clinical diagnosis is not always easy. The frequency of malignancy in thyroid nodules is reported as 5% and it is important to diagnose early because thyroid cancers progress slowly and a long survival is possible with early treatment (1, 2). Technical improvements in ultrasound (US) resulted with an increase in the rate of thyroid nodules that are not detected by a physical examination (3, 4). There are a vast number of studies about the role of gray scale (B-mode) US and power Doppler US (PDUS) in the diagnosis of malignant thyroid nodules. In these studies, it was concluded that PDUS itself or a combination of PDUS and B-mode US features were not as useful as the use of suspicious B-mode US features alone for predicting thyroid malignancy (4-13).
Recent studies have reported that spectral PDUS is useful in determining malignant nodules (1, 5). However, some authors state that vascular patterns or the resistive index (RI) values obtained by PDUS are not effective in differentiating benign and malignant nodules (1, 4, 11-13). To the best of our knowledge, previous studies did not analyze central and peripheral spectral Doppler vascular indexes separately (1, 5). In this study, we aimed to investigate the utility of PDUS and spectral Doppler vascular indexes in the diagnosis of benign-malignant thyroid nodules.
Seventy seven thyroid nodules were studied in 60 consecutive patients (20 men and 40 women) at our institution from July 2007 to December 2008. The patients ranged in age from 20 to 70 years (mean 49±11 years). Written informed consent from each subject was obtained and the ethics committee at our institute approved the procedures of this study.
All patients underwent a complete physical examination performed by two endocrinologists before US investigation. Symptoms and coexisting diseases of the patients were recorded. B-mode and duplex PDUS examinations were performed on all thyroid nodules prior to a fine needle aspiration biopsy (FNAB). Each nodule was evaluated three-dimensionally and the greatest diameter was recorded. All US and FNAB procedures were performed by an experienced radiologist.
Patients with the greatest nodule diameter (≥10 mm) observed by US examination were included in our study, prospectively.
Patients with undetermined, inadequate, or suspicious malignant cytology from FNAB, and patients who underwent FNAB before duplex PDUS were excluded from the study. Lastly, patients with hyperthyroidism or a hyperactive nodule, determined by thyroid scintigraphy, were excluded.
All US examinations and FNABs were performed with a 7.5 MHz linear-array transducer (Toshiba Applio System, Tokyo, Japan). Based on the number of nodules visualized, thyroid glands were classified as having a solitary nodule or as being multi-nodular. Each nodule was described according to diameter, inner structure, borders, parenchymal echogenicity, peripheral halo formation, and the presence of calcification (1, 5, 7, 14). The inner structure was described as solid, cystic or mixed (if the cystic component occupied an area of less than 25%, it was considered as solid; between 25 and 74% as mixed; and 75 and 100% as cystic). The nodule borders were described as smooth or irregular, with nodule calcification or no calcification, microcalcification (≤2 mm), central or peripheral calcification (>2 mm). The peripheral halo was described as present or absent. Lastly, echogenicity was described as hypoechoic, isoechoic, hyperechoic, or mixed when compared to the thyroid parenchyma (1, 15).
All nodules were examined by duplex PDUS to describe the vascular patterns. Nodules were identified as non-vascular, peripheral, central, or mixed (both peripheral and central vascularity) vascular according to visual evaluation (1, 16). Nodules without prominent vascularity were identified as non-vascular, while nodules with vascularity were identified as peripheral, central, or mixed vascular. RI and pulsatility index (PI) values were calculated from the central and peripheral arteries of thyroid nodules by standard software of our US equipment. The spectral Doppler parameters obtained were based on the formulas: PI = PSV (peak systolic velocity) - (mean diastolic velocity)/(mean velocity), and RI = PSV-mean diastolic velocity/PSV (5). RI and PI values were calculated from at least two central and two peripheral arteries from each nodule. For each nodule, the peripheral RI-PI and central RI-PI values were recorded separately, and the averages of the calculations were recorded. Also, mean RI-PI values were calculated. If there was no second artery to measure at the central or peripheral region of a nodule, a single measurement was taken into account.
Fine needle aspiration biopsy was performed under US-guidance for nodules sized ≥10 mm after duplex PDUS. FNAB examinations were performed twice for each nodule in the same session. Cytological material was smeared on slides immediately after aspiration and stained using the May-Grunwald-Giemsa stain (16). All slides were interpreted by an experienced cyto-pathologist who was blinded to the US findings. Less than six thyroid follicular cell groups were accepted as insufficient material and a FNAB was repeated. The final pathological diagnosis for each nodule was made by reports of US-guided FNAB or surgical pathological examination (if available) to categorize all nodules as benign or malignant.
Finally, a radiologist without knowledge of the clinical outcome independently reviewed each set of US images. The contribution of the physical examination, US findings, and FNAB results of all nodules to the final diagnoses were evaluated statistically.
Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL). Cyto-pathological results of each nodule were compared with physical-ultrasonographic examination findings and FNAB results, to examine the utility of these methods in the diagnosis of a malignancy. Before the comparison between malignant and benign groups, the Shapiro-Wilk normality test was applied in order to find out if the values of the variables were normally distributed. Student's t-test and the Mann-Whitney U test were used for comparison between groups. To determine the association among physical examinations, findings of US and cyto-pathological examinations underwent a Pearson Chi-Square test. Statistically significant B-mode US variables were analyzed by the binary logistic regression method. Receiver operating characteristic (ROC) curve analysis was performed to establish cut-off, sensitivity and specificity values associated with central, peripheral, and mean RI-PI values. Doppler indexes of non-vascular nodules could not be measured; therefore, such nodules were not counted in the statistical analysis concerning RI-PI values. Statistical significance was set at p < 0.05.
Mean patient age was 49.5 ± 9.7 and 49.3 ± 13.2 years in patients with benign and malignant nodules, respectively. Overall nodule diameter ranged from 10-42 mm in the greatest dimension (mean: 18 mm; standard deviation [SD]: 7 mm). Cyto-pathological results were similar in both sexes and different age groups (p > 0.05) (Table 1).
Co-morbid diseases were hypertension (13 cases), type 2 diabetes mellitus (5 cases), and coronary artery disease (2 cases). Compression symptoms such as hoarseness or trouble swallowing were apparent in 12 patients. Thyroid function tests were normal in 59 of 60 patients; subclinical hypothyroidism was diagnosed in one patient.
Upon physical examination of 77 nodules, 24 nodules were found to be hard, 24 were determined to be soft, and 29 were non-palpable. These 29 nodules were only noticeable under US examination. None of the nodules were fixed or painful. In one case, cervical adenopathy was recognized and FNAB of the nodule and adenopathy both revealed metastatic adenocarcinoma. Comparison of cytopathologic and physical examination findings revealed that the frequency of hard nodules in the malignant group was significantly higher compared to the benign group (p < 0.001) (Table 2).
A solitary nodule was found in 40 and multi-nodular goiter was found in 20 of 60 cases. No correlation was found between the cyto-pathological results and the number of nodules (p > 0.05).
Mean nodule diameter was 18.9 ± 7.4 mm in benign nodules and 17.2 ± 6.3 mm in malignant nodules (Fig. 1). Diameter, peripheral halo and inner structures of thyroid nodules were not distinctive for the diagnosis of malignancy (p > 0.05) (Table 2). A significant relationship was determined between malignancy and irregular margins on ultrasound examination (p < 0.05) (Table 2).
The relation between hypoechogenicity and malignancy was statistically significant (p < 0.05) (Fig. 1, Table 2). Risk of malignancy was 4 times higher in hypoechoic nodules when compared to iso-hyperechoic nodules (odds ratio: 4.01, 95% confidence interval [CI]: 1.207-13.294). The presence of a microcalcification was a significant indicator of malignancy (p < 0.05) (Table 2). A logistic regression analysis revealed calcification in general, as an independent risk factor for malignancy. The odds ratio of malignancy was 5.53 (95% CI: 1.556-19.663) for microcalcifications with a higher probability of malignancy.
According to PDUS findings, central, peripheral and mixed vascularity was seen in eight (11%), 23 (30%), and 42 (54%) nodules, respectively (Figs. 2, 3). A non-vascular appearance was evident in four (5%) nodules. Among the benign and malignant nodules, two (25%) and six (75%) showed central vascularity, respectively. Vascular patterns were not statistically different between the benign and malignant nodules (p = 0.054).
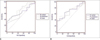
Central, peripheral, and mean RI-PI values were significantly higher in malignant nodules according to spectral Doppler US measurements (p < 0.05) (Fig. 4, Table 3). Sensitivity and specificity, negative/positive predictive values, cut-off values of RI-PI indexes for diagnosis of malignancy, and ROC curves are summarized in Table 4 and Figure 5.
Only six of the FNABs (8%) resulted in insufficient material. An additional FNAB performed on these six nodules revealed benign cytology. Eventually, 49 (64%) and 28 (36%) nodules were diagnosed as benign and malignant, respectively. There was no correlation between insufficient FNAB and nodule features or diameter (p > 0.05). The cyto-pathology of the 28 malignant nodules revealed papillary, follicular, medullary, anaplastic, and metastatic carcinoma. Cyto-pathological results of benign nodules showed a nodular colloid goiter and adenoma, with symptoms of Hashimoto's thyroiditis. A surgical procedure was performed on 18 malignant nodules, which revealed that the pathologic examinations were consistent with FNAB results. However, the FNAB results of one patient were interpreted as papillary carcinoma, but the pathologic result was reported as a follicular variant of papillary carcinoma. Patients with a benign cytology were not operated on, but were followed up.
It is of utmost importance to recognize absolutely reliable criteria for malignancy when using imaging methods (17). The use of US in the assessment of thyroid disease has greatly increased the ability to detect small thyroid nodules, which were unrecognized by clinical examinations (7). Neither US nor FNAB has yielded satisfactory diagnostic accuracy for thyroid cancer (13). The correct diagnosis is established by means of a histopathologic examination (12, 17). On the other hand, management for thyroid nodules is now controversial (2, 18, 19). The question of whether clinically unapparent thyroid lesions should be assessed by FNAB is still unresolved (7). According to the literature, it is expensive and impractical to perform a FNAB on all nodules found incidentally. It is suggested in previous guidelines and Ultrasound Consensus Conferences that for nodules less than 10 mm, only those with a high-risk of malignancy or suspicious US features must be biopsied (9, 18, 19).
There are a vast number of studies about the role of B-mode US and PDUS in the diagnosis of malignant nodules (1-10, 15-27). These studies state that hypoechogenicity, microcalcification, and margin irregularity of thyroid nodules are important features in determining the malignancy risk, as observed in our study. Also, the relationship between hard nodule and malignancy was statistically significant in our study. Recently, one study showed evidence of the relationship between hypoechogenicity and fibrosis in papillary thyroid carcinoma (28). Hypoechogenicity and hard nodules are related to the fibrosis of papillary thyroid carcinoma (28).
Recently, it was suggested that parameters identified by spectral Doppler US may be beneficial to differentiate between malignant and benign nodules (1, 5). Although some other studies report contradictory results (4, 11, 12), thyroid nodules are classified as non-vascular, peripheral vascular, central vascular, and mixed vascular according to the PDUS examination findings (1, 2, 5, 10, 15). Generally, increased central vascularity is accepted in the literature as a supporting feature for diagnosis of malignancy (1, 7, 10, 27, 29). In our study, we did not find any relationship between central vascularity and malignancy. Moon et al. (13) also suggested the intranodular vascularity is not a feature of malignancy. Shimamoto et al. (30), Argalia et al. (31), and Tamsel et al. (16), also did not find any relationship between intratumoral vascularity and malignancy, which is consistent with the results of our study. Improvements in US technology may result in the early diagnosis of small thyroid nodules, in which central vascularity is not apparent. De Nicola and colleagues (32) emphasized that absence of central vascularity cannot exclude malignancy. Bakhshaee et al. (5) described in their study that more than 90% of malignant nodules are mixed vascular and none of them have only central vascularity. In summary, subjective findings such as increased central or peripheral vascularity are not reliable tools for thyroid nodule evaluation (12, 13, 33).
The sensitivity of Doppler US investigation is affected by the technical parameters as settings of a wall filter, nodule depth, and pulse repetition frequency (PRF) (1, 5, 32). Individual variations of tissue attenuation, patient movement and lack of cooperation, motions as swallowing or breathing and pulsations of adjacent arterial structures may affect Doppler US investigation (1, 32). Over-pressing thyroid tissue may also prevent detecting vascularity (5). An operator must consider these points and use an appropriate technique. We assume that the technical issues mentioned above might have interacted and hid the possible relationship between intranodular vascularity and malignancy in our study.
Assessment of thyroid nodules by RI and PI values measured by spectral Doppler US is not affected from course of artery, angle of insonation, or nodule size (1, 2, 5, 16). Contrarily, blood velocity measurements may be altered by Doppler parameters chosen by radiologists (1, 2, 5, 16). Many studies investigated blood velocity parameters in the diagnosis of thyroid cancer and generally it is not considered useful (1, 2, 16). In light of these data, we analyzed only RI and PI values instead of velocity measures in our study.
In our study, we found that malignant nodules have significantly higher RI and PI values according to benign nodules. Other studies by Argalia et al. (31), De Nicola et al. (32), Ivanac et al. (34) and Yang et al. (35) also reported similar results for RI values. Bakhshaee and colleagues (5) reported mean PI and RI values of 1.15 ± 0.33 and 0.72 ± 0.13, respectively in malignant nodules. Chammas and colleagues (1) described the same values of 1.53 ± 0.63 and 0.74 ± 0.12, respectively. In our study, the RI values of malignant nodules were compatible with the literature and the PI values were a bit higher, but quite similar according to the literature. It can be postulated that stenosis and/or occlusion of arteries due to excess cellular proliferation in malignant nodules might occur as a result of the high central and peripheral RI-PI values noted in our study (1, 5). It is already known that low diastolic-high systolic flow observed in stenotic vessels causes high RI and PI values in malignant nodules (1, 5).
There are two studies in the literature investigating the utility of RI and PI values for the diagnosis of malignant thyroid nodules (1, 5). Both studies calculated RI-PI values from the central and peripheral regions of each nodule; the average RI-PI values were accepted as actual values (36). Instead, we calculated vascular indexes of central and peripheral arteries separately. Although the sensitivity and negative predictive values of the RI-PI values measured at central and peripheral are higher than the mean RI-PI values, we detected that the specificity and positive predictive values of the mean PI-RI values are higher than the values measured in the central and peripheral regions. The sensitivity and specificity of central, peripheral, and mean RI-PI values was different (Table 4); hence, the assessment of all these values together may be useful in the differentiation of benign and malignant nodules. To our knowledge, our study is the first with this feature. We believe the influence of these vascular indexes should be tested with further comprehensive studies with a larger patient cohort.
There are several potential limitations of this study. The main limitation is that pathologic examination results were not available for all nodules. In B-mode US; we determined there to be high RI-PI values in two nodules with a smooth border and also in one hyperechoic nodule. The pathologic examination of these cases resulted in the diagnosis of malignant nodules. We did not compare all gray scale US and PDUS findings in all nodules. This is the second limitation of our study. Assessment of vascular patterns by PDUS is an inevitably subjective method. Examination of each nodule with B-mode US and duplex PDUS by different radiologists and investigation of intra- and inter-observer agreement will be necessary in further studies.
In conclusion, both gray scale US and PDUS have their own strengths and weaknesses. We can simply speculate from all previous literatures that B-mode US and PDUS might complement each other in the differentiation of benign and malignant nodules. RI and PI values are useful in distinguishing malignant thyroid nodules from benign ones.
Figures and Tables
Fig. 1
Mixed vascular, solid and hypoechoic nodule diagnosed with papillary carcinoma. Pulsatility index and resistive index values calculated by spectral Doppler US were 1.15-0.73 (A) and 1.3-0.76 (B) in center and periphery of nodule, respectively. In C, characteristic papillary formation of carcinoma cells with nuclear grooving and nuclear clearing was seen.
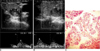
Fig. 2
Mixed vascular, solid, and hypoechoic benign nodule (A). Nodule was diagnosed as nodular goiter by fine needle aspiration biopsy. Pulsatility index and resistive index values calculated by spectral Doppler US were 0.91-0.50 (B) and 0.73-0.53 (C) in central and peripheral section of nodule, respectively.
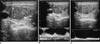
Fig. 3
Hypoechoic, solid nodule with mixed vascular pattern observed using power Doppler US without halo and calcification (result of fine needle aspiration biopsy was nodular hyperplasia). Pulsatility index and resistive index values calculated by spectral Doppler US were 1.29-0.69 (A) and 0.82-0.51 (B) in central and peripheral sections of nodule, respectively. Pigmented histiocyte and follicular epithelial cells are seen in this sample of cystic nodular hyperplasia (May-Grünwald-Giemsa staining, ×200) (C).
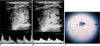
Fig. 4
Hypoechoic, solid nodule with smooth borders. Although there is no halo or calcification, it was diagnosed as medullary carcinoma. It has mixed vascular pattern in power Doppler US. Pulsatility index and resistive index values calculated by spectral Doppler US were 1.26-0.73 (A) and 1.63-0.80 (B) in center and periphery of nodule, respectively. Isles of cells with large nuclei and wide granular cytoplasm showed marked pleomorphism (C).
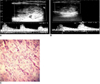
Fig. 5
Receiver operating characteristic (ROC) curves of resistive index (RI) (A) and pulsatility index (PI) (B) indexes.
References
1. Chammas MC, Gerhard R, de Oliveira IR, Widman A, de Barros N, Durazzo M, et al. Thyroid nodules: evaluation with power Doppler and duplex Doppler ultrasound. Otolaryngol Head Neck Surg. 2005. 132:874–882.
2. Fukunari N, Nagahama M, Sugino K, Mimura T, Ito K, Ito K. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg. 2004. 28:1261–1265.
3. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003. 22:1083–1090.
4. Iannuccilli JD, Cronan JJ, Monchik JM. Risk of malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004. 23:1455–1464.
5. Bakhshaee M, Davoudi Y, Mehrabi M, Layegh P, Mirsadaee S, Rad MP, et al. Vascular pattern and spectral parameters of power Doppler ultrasound as predictors of malignancy risk in thyroid nodules. Laryngoscope. 2008. 118:2182–2186.
6. Decherd ME, Ryan MW. Evaluation of the thyroid nodule. Grand Rounds Presentation, UTMB, Dept. of otolaryngology. 2002.
7. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 87:1941–1946.
8. Peccin S, de Castsro JA, Furlanetto TW, Furtado AP, Brasil BA, Czepielewski MA. Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest. 2002. 25:39–43.
9. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005. 237:794–800.
10. Appetecchia M, Solivetti FM. The association of colour flow Doppler sonography and conventional ultrasonography improves the diagnosis of thyroid carcinoma. Horm Res. 2006. 66:249–256.
11. Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003. 22:1027–1031.
12. Stacul F, Bertolotto M, De Gobbis F, Calderan L, Cioffi V, Romano A, et al. US, colour-Doppler US and fine-needle aspiration biopsy in the diagnosis of thyroid nodules. Radiol Med. 2007. 112:751–762.
13. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010. 255:260–269.
14. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006. 91:3411–3417.
15. Gul K, Ersoy R, Dirikoc A, Korukluoglu B, Ersoy PE, Aydin R, et al. Ultrasonographic evaluation of thyroid nodules: comparison of ultrasonographic, cytological, and histopathological findings. Endocrine. 2009. 36:464–472.
16. Tamsel S, Demirpolat G, Erdogan M, Nart D, Karadeniz M, Uluer H, et al. Power Doppler US patterns of vascularity and spectral Doppler US parameters in predicting malignancy in thyroid nodules. Clin Radiol. 2007. 62:245–251.
17. Chammas MC, de Araujo Filho VJ, Moysés RA, Brescia MD, Mulatti GC, Brandão LG, et al. Predictive value for malignancy in the finding of microcalcifications on ultrasonography of thyroid nodules. Head Neck. 2008. 30:1206–1210.
18. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.
19. Gharib H, Papini E, Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008. 159:493–505.
20. Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995. 141:259–277.
21. Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age and multinodularity. Am J Med. 1992. 93:363–369.
22. Franklyn JA, Daykin J, Young J, Oates GD, Sheppard MC. Fine needle aspiration cytology in diffuse or multinodular goitre compared with solitary thyroid nodules. BMJ. 1993. 307:240.
23. Schneider AB, Bekerman C, Leland J, Rosengarten J, Hyun H, Collins B, et al. Thyroid nodules in the follow-up of irradiated individuals: comparison of thyroid ultrasound with scanning and palpation. J Clin Endocrinol Metab. 1997. 82:4020–4027.
24. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994. 154:1838–1840.
25. Tomimori E, Pedrinola F, Cavaliere H, Knobel M, Medeiros-Neto G. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid. 1995. 5:273–276.
26. Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992. 20:37–42.
27. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007. 100:29–35.
28. Jung SL, Jung CK, Kim SH, Kang BJ, Ahn KJ, Kim BS, et al. Histopathologic findings related to the indeterminate or inadequate results of fine-needle aspiration biopsy and correlation with ultrasonographic findings in papillary thyroid carcinomas. Korean J Radiol. 2010. 11:141–148.
29. Cappelli C, Pirola I, Cumetti D, Micheletti L, Tironi A, Gandossi E, et al. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-needle aspiration cytology? Clin Endocrinol (Oxf). 2005. 63:689–693.
30. Shimamoto K, Sakuma S, Ishigaki T, Makino N. Intratumoral blood flow: evaluation with color-Doppler echography. Radiology. 1987. 165:683–685.
31. Argalia G, D'Ambrosio F, Lucarelli F, Mignosi U, Giuseppetti GM, Passarini G, et al. Echo Doppler in the characterization of thyroid nodular disease. Radiol Med. 1995. 89:651–657. [Italian].
32. De Nicola H, Szejnfeld J, Logullo AF, Wolosker AM, Souza LR, Chiferi V Jr. Flow pattern and vascular resistive index as predictors of malignancy risk in thyroid follicular neoplasms. J Ultrasound Med. 2005. 24:897–904.
33. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009. 19:1923–1931.
34. Ivanac G, Brkljacic B, Ivanac K, Huzjan R, Skreb F, Cikara I. Vascularisation of benign and malignant thyroid nodules: CD US evaluation. Ultraschall Med. 2007. 28:502–506.
35. Yang TF, Wang JD, Luo HJ, Wang XY, Li FH. Relationship between ultrasonographic velocimetric parameters and microvessel density in patients with papillary thyroid carcinoma and its clinical significance. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007. 42:126–129. [Chinese].
36. Algin O. Spectral power Doppler ultrasound parameters: are they really significant? Laryngoscope. 2009. 119:1452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download