Abstract
Although liver transplantation (LT) is the only effective treatment option for hepatopulmonary syndrome (HPS), the post-LT morbidity and mortality have been high for patients with severe HPS. We performed post-LT embolotherapy in a 10-year-old boy who had severe type I HPS preoperatively, but he failed to recover early from his hypoxemic symptoms after an LT. Multiple embolizations were then successfully performed on the major branches that formed the abnormal vascular structures. After the embolotherapy, the patient had symptomatic improvement and he was discharged without complications.
Hepatopulmonary syndrome (HPS) is a pulmonary vascular disorder that is characterized by the clinical triad of chronic liver disease, arteriovenous shunts due to intrapulmonary vasodilatation, and arterial hypoxemia (1, 2). The pathophysiological mechanisms associated with HPS have not been identified. Although HPS is most commonly found in patients with cirrhosis, it has also been seen in patients with portal hypertension in the absence of cirrhosis, and HPS has also been reported in the setting of acute and chronic hepatitis in the absence of portal hypertension (1, 3). The diagnosis of HPS is made by identifying the intrapulmonary shunts in those patients with liver disease and hypoxemia. The shunts can be qualitatively identified by performing contrast echocardiography and they can also be quantitatively measured by performing 99m-Tc macroaggregated albumin (MAA) lung perfusion scanning (1-5).
There is currently no effective medical treatment for HPS. Liver transplantation (LT) is considered as the only mainstay of therapy for HPS (1, 2). However, HPS may not be reversible even after an LT in severe cases. A low preoperative partial pressure of arterial oxygen (PaO2) and a large amount of shunting have been reported to be associated with high post-LT morbidity and mortality (1).
We performed living donor LT in a patient with severe type I HPS (PaO2 = 39.7 mmHg). Although the liver function rapidly normalized, the patient's hypoxemia and dyspnea were not improved one month after the LT. Therefore, embolization of the intrapulmonary shunts was attempted in an effort to reduce the amount of shunting. The patient's symptoms dramatically improved after the procedure.
The patient was a 10-year-old boy. A Kasai operation was performed for treating his biliary atresia at three months after he was born. Although he had received endoscopic ligation for grade III esophageal varices at the age of four because of intermittent hematemesis and melena, he had maintained good general health and hepatic function for a long time after the Kasai operation. However, he complained of dyspnea on exertion at eight years of age. His symptoms continued for several months; the patient was closely followed during this time to determine the cause of the dyspnea. Saline contrast echocardiography showed intrapulmonary right to left shunts and so the diagnosis of HPS was made. The 99mTc-MMA lung perfusion scanning showed 45% shunting, with assuming that 13% of the cardiac output is delivered to the brain (Fig. 1A). The arterial oxygen saturation (SaO2) at the time of diagnosing HPS was 84% on the pulse oximetry monitoring. The patient's symptoms gradually worsened. One year later, the PaO2 was as low as 37.3 mmHg and the SaO2 was only 71% in room air. The patient's activity was much decreased. An LT was recommended for the serious symptoms associated with the HPS, despite the relatively tolerable hepatic function.
The patient developed fever and a headache while waiting for an LT. Two rim-enhanced cystic lesions in the left frontal and parietal lobes were detected on the brain computed tomography (CT) scan. Abscess removal via a craniotomy was performed after 8-weeks of antibiotic therapy. The infection was completely controlled, yet no deceased donor graft was available for him. The patient's general activity continued to worsen; his physical activity was markedly decreased. Thus, a living donor LT was performed with the liver obtained from the patient's father. At the time of the LT, the hepatic function was relatively good compared to the patient's general condition. The serum total bilirubin and albumin levels were 1.8 mg/dL and 3.4 g/dL, respectively. The international normalized ratio (INR) for the prothrombin time was 1.35. The Child-Pugh score was 6 and the model for end-stage liver disease (MELD) score was only 2. However, his PaO2 and SaO2 were still very low in room air (39.7 mmHg and 73%, respectively). The CT angiography showed diffuse peripheral pulmonary vasodilatation (Fig. 1B).
The LT was performed using an extended left liver graft from the patient's father. The graft included the middle hepatic vein and the graft-to-recipient weight ratio (GRWR) was 1.47. After the LT, the hepatic function rapidly normalized by 10 days post surgery. However, the respiratory symptoms did not improve even after one month post surgery. The dyspnea was more severe than that prior to the LT. The respiratory rate was 24 to 30 per minute and the SaO2 was only 50 to 60% on the pulse oximetry monitoring with facial mask O2 supplementation of 8 L/min. The patient continued to have very limited physical activity and he was bed-ridden.
Thirty days post surgery, the first embolization was attempted in the left lung. The angiography showed abnormal tortuous and dilated vascular structures in the left upper lung field. Multiple embolizations were performed on the major branches that formed the abnormal vascular structures by using 8 MicroNester® Embolization Microcoils™ 4 mm/14 cm (Cook, Bloomington, IN) (Fig. 1E, F). Right after the first embolization, the abnormal vascular structures were markedly decreased and the SaO2 was increased by 10%. The patient's symptoms were slightly improved. The respiratory rate decreased to 20 to 24 per minute and the SaO2 increased to 65 to 75% on monitoring with facial mask O2 supplementation of 5 L/min. The second embolization was performed one week later in the right lung. On the second angiography, the abnormal dilated vascular structures were found mainly in the right lower lung field. Multiple embolizations were performed with 9 coils in the same manner (Fig. 1E, F). After the embolotherapy, the patient's activity level gradually improved and he was discharged, with O2 supplementation of 5 L/min, three days after the second embolization. Wheel-chair ambulation was possible for about ten minutes without O2 at the time of discharge.
At three months post-LT, the patient was admitted with seizures that were caused by an inactive brain lesion related to the previous surgery. The seizures were treated without complication. At that time, his PaO2 and SaO2 were 65 mmHg and 93%, respectively, on room air and his activity had markedly improved; he played as actively as the other children in his age group. The patient was doing well with normal liver function at the two year follow up.
Hepatopulmonary syndrome is defined by a widened alveolar-arterial oxygen gradient (P[A-a]O2) that is the result of intrapulmonary vasodilatation in the presence of hepatic dysfunction or portal hypertension (5). The diagnosis of HPS can be variable based on the numeric threshold that is used for hypoxemia (2). In general, the cases with delayed positive-contrast echocardiography (the left atrial microbubble opacification > 3 beats after the right atrial opacification) as well as hypoxemia, with a PaO2 < 70 mmHg or P(A-a)O2 > 20 mmHg, are considered to have HPS. HPS is found in 8 to 17% of the patients with cirrhosis (4, 6). The clinical features of HPS typically involve respiratory complaints. Platypnea and orthodexia are classically presented; they are highly specific signs for HPS in the setting of liver cirrhosis, and they are the result of a gravitational increase in blood flow through the dilated vessels in the lung bases. Cough, clubbing and distal cyanosis can also be associated with HPS (3).
In patients with the HPS, enhanced pulmonary production of nitric oxide (NO) has been implicated in the development of intrapulmonary vasodilatation (5). Acute inhibition of NO production or its action with administering NG-nitro-L-arginine methyl ester (L-NAME) or methylene blue, respectively, transiently improves HPS (2, 3, 5). However, all the medical treatments used to date have been disappointing over the long-term (2, 3, 5). LT is the only effective method for the total resolution or marked improvement of HPS (5). Post-LT significant improvement of gas exchange has been reported in more than 80% of the patients with HPS (7). Yet the length of time it takes for the hypoxemia to normalize after LT varies and this may be more than one year (5). In addition, the post-LT morbidity and mortality are increased in patients with severe HPS, as compared to those who do not have HPS and who have undergone LT (1, 5). For patients with a low PaO2 less than 50 mmHg, the post-LT survival has been reported to be lower than that for other patients (5). Arguedas and colleagues (6) prospectively evaluated the post-LT outcomes and predictive factors of patients with HPS. According to their report, for the room air PaO2 level, a threshold value of ≤ 50 mmHg had the best predictive value for post-LT mortality with a sensitivity of 86% and a specificity of 82%; a threshold value of ≥ 20% for the MMA shunt fraction had the best predictive value with a sensitivity of 100% and a specificity of 76%. The combination of the room air PaO2 ≤ 50 mmHg and an MMA shunt fraction ≥ 20% further increased the predictive value with a sensitivity of 86% and a specificity of 88%. Among the patients who met these two criteria, three fourths died after LT (a positive predictive value of 75%). Therefore, although LT is the best treatment modality for HPS, LT should be performed with caution in patients with severe HPS because of the high post-LT mortality.
Embolotherapy is not an established therapy for the type I HPS. The type II HPS with large pulmonary arteriovenous fistulas can be easily treated by embolizations (2, 8, 9). Some authors believe that coil embolization is the first line of therapy for the type II HPS, followed by considering performance of LT (9). However, the type II HPS is not a common type. Most patients with the HPS show type I, which is characterized by diffuse precapillary pulmonary artery dilatation without any definite arteriovenous fistulas. Embolotherapy for the type I HPS is more difficult and it is still unreliable as an effective treatment method. There are limited case reports of embolizations for type I HPS. Ryu and Oh (10) first reported successful diffuse embolization as a rescue therapy for a patient with the type I HPS and who was waiting for an LT. They selected the main branches forming the abnormal tortuous vascular structures and they performed multiple embolizations at the level of the segmental arteries. They reported that the patient had symptomatic improvement and he maintained his respiration with O2 supplementation of 1 L/min, although the long-term outcome is unknown. Saad et al. (9) also reported successful post-LT pulmonary arteriolar embolizations for the type I HPS, which were performed to reduce the morbidity during the postoperative period. However, there is little accumulated experience with embolotherapy for the type I HPS. Thus, its efficacy and risks have not yet been established.
The patient reported on here had severe HPS preoperatively. The laboratory hepatic function was completely normal by 10 days after the surgery. However, symptomatic improvement was not achieved at one month post-LT, and the patient's general physical activity further deteriorated. Delayed improvement of hypoxemia has been previously reported (5), and the post-LT mortality has been reported to be very high for such patients (1, 6). Moreover, the long duration of inactivity could led to other complications such as pneumonia and bedsores.
Despite that our patient had the type I HPS, multiple embolizations were successfully performed on the major arterioles forming the abnormal tortuous and dilated vascular structures. The patient could be discharged with improved symptoms after embolotherapy and two months later there was nearly normal arterial oxygenation and active movement when breathing room air. Therefore, post-transplant embolotherapy for the type I HPS might be effective and safe in patients who have severe HPS preoperatively and who fail to recover early from their hypoxemic symptoms. Although this treatment modality cannot completely resolve the type I HPS, it can accelerate the post-LT recovery and decrease the risk of postoperative morbidity and mortality by reducing the amount of shunting.
In conclusion, although LT is the only established effective therapy for HPS that can totally resolve or markedly improve HPS, the patients with severe HPS may have sustained hypoxemic symptoms for a long time after an LT; such patients have a high risk of postoperative morbidity and mortality. We found that embolotherapy could improve the symptoms by reducing the amount of shunting even in a case with the type I HPS. Therefore, post-transplant pulmonary artery embolization might be a useful treatment option to accelerate the post-LT recovery and to decrease the risk of morbidity and mortality in patients who fail to recover early after an LT. Further study is needed to confirm these findings.
Figures and Tables
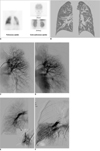 | Fig. 1Embolotherapy for hepatopulmonary syndrome in 10-year-old boy.
A. 99m-Tc macroaggregated albumin lung perfusion scanning. Large proportion of radioactive substance was detected in extra-pulmonary areas; mainly brain and kidneys. Amount of intrapulmonary shunting was 45% with assuming that 13% of cardiac output is delivered to brain.
B. Pre-transplant pulmonary CT angiography shows diffuse peripheral pulmonary vasodilatation.
C. Pre-embolization angiography shows abnormal tortuous and dilated vascular structures in left upper lung field.
D. We performed embolization with 8 coils by selecting major branches that formed abnormal vascular structures. Right after embolization, we noted marked decrease in size of abnormal vascular structures and SaO2 had increased by 10%.
E. Pre-embolization angiography shows abnormal dilated vascular structures were mainly located in right lower lung field.
F. We performed embolization with 9 coils. Patient was discharged three days after second embolization with O2 supplementation of 5 L/min.
|
References
1. Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology. 2005. 41:1122–1129.
2. Mandell MS. The diagnosis and treatment of hepatopulmonary syndrome. Clin Liver Dis. 2006. 10:387–405.
3. Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol. 2006. 45:617–625.
4. Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000. 118:615–624.
5. Arguedas MR, Fallon MB. Hepatopulmonary syndrome. Clin Liver Dis. 2005. 9:733–746.
6. Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003. 37:192–197.
7. Lange PA, Stoller JK. The hepatopulmonary syndrome. Effect of liver transplantation. Clin Chest Med. 1996. 17:115–123.
8. Poterucha JJ, Krowka MJ, Dickson ER, Cortese DA, Stanson AW, Krom RA. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology. 1995. 21:96–100.
9. Saad NE, Lee DE, Waldman DL, Saad WE. Pulmonary arterial coil embolization for the management of persistent type I hepatopulmonary syndrome after liver transplantation. J Vasc Interv Radiol. 2007. 18:1576–1580.
10. Ryu JK, Oh JH. Hepatopulmonary syndrome: angiography and therapeutic embolization. Clin Imaging. 2003. 27:97–100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download