Abstract
Objective
To describe detailed clinical and radiological features of the pandemic H1N1 2009 influenza viral infection among healthy young males in a semi-closed institutionalized setting.
Materials and Methods
A total of 18 patients confirmed with the pandemic H1N1 2009 influenza virus infection from July 18 to July 30, 2009 were enrolled in this study. Each patient underwent an evaluation to determine detailed clinical and radiological features.
Results
All patients presented with high fever (> 38.0℃), with accompanying symptoms of cough, rhinorrhea, sore throat, myalgia and diarrhea, and increased C-reactive protein (CRP) values with no leukocytosis nor elevated erythrocyte sedimentation rate (ESR). All patients, including one patient who progressed into acute respiratory distress syndrome, were treated with oseltamivir phosphate and quickly recovered from their symptoms. Chest radiographs showed abnormalities of small nodules and lobar consolidation in only two out of 18 patients. However, six of 12 patients who underwent thin-section CT examinations showed abnormal findings for small ground-glass opacities (GGOs) in addition to poorly-defined nodules with upper lobe predominance.
In March and early April 2009, there was an outbreak of influenza in Mexico, which was confirmed to be caused by the pandemic H1N1 2009 influenza virus. Soon thereafter, cases began to be reported in other countries in North America, Europe, South-East Asia, and Australia. As of August 6, 2009, over 170 countries and territories have reported cases, with a total of 343,298 confirmed cases and 4,108 deaths worldwide (1). On June 11, 2009, the World Health Organization (WHO) raised the level of pandemic alert from phase 5 to 6, which is defined as a new virus causing sustained community level outbreaks in more than one WHO region (2).
In late July of 2009, there was an outbreak of the pandemic H1N1 2009 influenza virus infection in a South Korean military barrack. All patients were previously healthy young soldiers. Although several papers (3-13) have been published about the clinical features of the pandemic H1N1 2009 influenza virus infection among an unspecified population, there have been no reports regarding this infection among a specific subset of either an institutionalized or semi-closed population setting, such as in military barracks composed of healthy young adults, where a large number of individuals live in close contact and have higher reported rates of viral infections (14). Furthermore, there have been a few reports providing detailed descriptions of the radiologic features of the pandemic H1N1 2009 influenza virus infection such as in thin-section CT (11-13).
In this article, we aim to describe detailed clinical characteristics and radiologic features of chest radiographs and thin-section CT findings of patients confirmed with the pandemic H1N1 2009 influenza virus infection occurring in a semi-closed setting.
This study was approved by the ethics committee of the Armed Forces Medical Command, which waived the requirement of informed patients consent for this retrospective study.
From July 18 to July 30, 2009, 18 out of 57 patients who presented with acute febrile respiratory illness (fever of 38℃ or higher, and/or a cough or shortness of breath) (8), were confirmed with the pandemic H1N1 2009 influenza virus infection in a tertiary military hospital in South Korea. We retrospectively reviewed the medical charts, as well as the laboratory and radiologic findings of these patients.
All patients were young male soldiers with a mean age of 20 years (age range: 19 to 22 years). Fifteen of the 18 patients confirmed with the pandemic H1N1 2009 influenza virus infection were from the same barrack, and they visited the emergency department due to acute febrile respiratory illness from July 18 to July 20, 2009. The other three patients were from different barracks and were admitted with suspected cases of rhabdomyolysis after a long distance march (n = 1), acute gastroenterocolitis (n = 1), and acute febrile respiratory illness with leukocytopenia and thrombocytopenia (n = 1). All three patients came into contact with one or more of the previously-mentioned patients lying in nearby beds in our hospital.
Among the 15 patients who presented to the emergency department with acute febrile respiratory illness, two patients had returned from a brief visit to their hometowns one week prior to the outbreak of acute febrile respiratory illness and were suspected as the source of the illness.
Specimens from a nasopharyngeal swab (n = 17), and tracheal aspirate (n = 1) were collected at the emergency department or wards when the 15 patients of the same barrack visited the emergency department, or when symptoms developed in the remaining three patients who came into contact with them. Reverse-transcriptase-polymerase-chain-reaction (RT-PCR) tests and additional viral cultures were performed in accordance with published guidelines from the WHO in all patients (8).
A malaria smear and triple antibody tests of Hantann, leptospirosis, and Tsutsugamushi virus were also performed to rule out other potential causes of fever, and revealed no remarkable abnormalities.
Two authors retrospectively reviewed the clinical and laboratory findings. For the review of the clinical features, initial symptoms such as fever, cough, sputum, rhinorrhea, sore throat, myalgia, headache, dizziness, vomiting, and diarrhea were recorded. In turn, patient symptom progression while under treatment was also reviewed. For review of the laboratory data, the hematological analysis of the white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin level, hematocrit level, platelet count, neutrophil count and the biochemical analysis of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were recorded.
Chest radiographs and thin-section CT examinations were obtained from 18 and 12 patients, respectively, prior to the administration of antiviral agents. Two out of the 12 patients undergoing thin-section CTs received follow-up thin-section CT examinations a week after starting antiviral therapy.
All radiographic examinations were performed using digital radiographic equipment (TDR4600-F80; Gold Mountain Medical System, Seoul, Korea) and a standardized technique (110 kV, 2 mAs, and a 180-cm film-focus distance for the posteroanterior views). Thin-section CTs of the thorax were performed using a 16 channel CT scanner (BrightSpeed; GE Medical Systems, WI), with the following parameters: 1.25 mm slice thickness with a 2.5 mm gap, supine position, scanning during inspiration, 6 seconds scan time, 120kV, auto mA.
All chest radiographs and thin-section CT images were reviewed by three experienced chest radiologists (8, 10, and 16 years of experience in chest radiology, respectively) using a picture archiving and communication systems (PACS) viewer. Decisions were reached by consensus.
To review chest radiographs, the presence of nodular opacity, consolidation (focal, multifocal, or bilateral) or interstitial patterns, distribution of findings including central (inner two-thirds of lung) or peripheral (outer one-third of lung) and upper, middle or lower lung zones, atelectasis, mediastinal abnormalities, and the presence or absence of a pleural effusion were analyzed (15).
Thin-section CT findings were interpreted using the descriptors proposed by the Fleischer Society Nomenclature Committee (16). Ground-glass opacity (GGO) was defined as an increase in the lung parenchymal attenuation that did not obscure the underlying vascular architecture. A nodule was defined as round opacity, at least moderately well-defined, and no more than 3 cm in diameter. Consolidation was defined as increased lung attenuation that obscured the underlying vasculature (16). The lesion size was described as small (diameter, less than 1 cm), medium (diameter, 1 to 3 cm), large (diameter, 3 cm to 50% of the segment), or segmental (50% to 100% of the segment). The sites were described by the name and number of involved segments of the lung. Location of the lesion was defined as peripheral if it was in the outer one-third of the lung; otherwise, it was defined as central (17). Attention was also paid to the presence of other abnormalities such as pleural effusion, lymphadenopathy, cavitation, calcification, or septal thickening.
The clinical course and outcome of all patients are summarized in Figure 1. In the analysis of 15 patients who visited the emergency department, the mean interval between symptom onset and emergency room visit was 1.7 days ± 1.5 days (range, 0 to 5 days). In the analysis of all 18 patients, the mean interval between symptom onset and the start of oseltamivir phosphate (Tamiflu®, Roche, Basel, Switzerland) administration was 3.9 ± 1.6 days (range, 1 to 7 days). In addition, the mean interval between symptom onset and symptom improvement was 4.7 ± 1.7 days (range, 2 to 8 days).
The clinical characteristics of the 18 patients in our study are summarized in Tables 1 and 2. All patients presented with high fever (> 38.0℃, median value and range; 38.5℃ and 38.2-39.5℃), with common accompanying symptoms including coughing (78%), rhinorrhea (89%), sore throat (78%), myalgia (78%), vomiting (50%) or diarrhea (56%). A laboratory analysis revealed that none of the patients had leukocytosis. Seventeen out of the 18 patients showed WBC counts within normal limits, whereas one patient showed leukocytopenia. In addition, thrombocytopenia and lymphocytopenia were present in three of 18 (17%) and 12 of 18 patients (67%), respectively. No abnormality was noted for the RBC count, hemoglobin level, hematocrit level, platelet count, and neutrophil count. The AST or ALT was abnormal in three and one patient(s), respectively. ALP was within normal range for all patients. CK was elevated in seven of 18 patients (39%). ESR was within the normal range in all patients. However, all patients showed elevated CRP values.
After the diagnosis of the pandemic H1N1 2009 influenza virus infection through RT-PCR tests and additional viral cultures, all patients were treated with oseltamivir phosphate. Of the 18 patients, 16 rapidly recovered after the administration of the antiviral agent. In the two remaining patients, the symptoms started to improve prior to oseltamivir phosphate treatment.
Among our study population, one patient presented with high fever, severe nocturnal cough, and blood tinged sputum, and the respiratory symptoms eventually progressed into acute respiratory distress syndrome (ARDS), which further led him to receive mechanical ventilation (case No. 12). A retrospective medical chart review revealed that this patient was a solider serving in a different unit from the majority of the other patients and had been in contact with a patient with pandemic H1N1 2009 influenza virus infection lying in a nearby bed in our hospital prior to symptom aggravation. The laboratory data of this patient showed leukocytopenia, thrombocytopenia, and an increased CK level. Other laboratory tests were within normal limits. He was initially treated with antibiotics such as macrolide and a 3rd generation cephalosporin under suspicion of bacterial pneumonia, which was never confirmed. However, his symptoms did not improve. Five days after contact with a patient with pandemic H1N1 2009 influenza viral infection, severe respiratory distress presented and a laboratory analysis from the tracheal aspirate performed during endotracheal intubation confirmed the pandemic H1N1 2009 influenza virus infection. Following the administration of oseltamivir phosphate and antibiotics, the patient's symptoms improved and was taken off mechanical ventilation five days after antiviral agent administration.
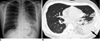
Sixteen out of 18 patients in our study population showed no abnormalities on chest radiographs. Moreover, only two patients in our study showed abnormal findings of a small central nodular opacity in the right upper lung zone in one (Fig. 2A), and consolidations in the left middle lung and both lower lungs in the other (Fig. 3A) on chest radiographs.
On thin-section CT images, six of 12 patients (50%) showed abnormal findings. Of these six patients, five (83%) had small GGOs; four patients had them in the upper lobe of the lung and one had a small GGO discovered in the lower lobe of the lung. Also, in four out of the five patients with small GGOs (80%), the lesions were localized in the periphery of the lung, and two of the five patients also showed bilateral involvement. For segmental involvement in terms of lesion size, three patients showed one segment, one patient showed two segments, one patient showed three segments, and one patient showed eight segments. Nodules were observed in all patients (100%), which were small in size with showing poorly defined margins (Fig. 2B). In five patients (83%), the nodules were combined with GGOs in the same area. In addition, the nodule was a solitary finding in one patient (17%), which was located in the peripheral area of the right upper lobe. The patient who progressed into ARDS had bilateral lobar consolidations combined with GGOs, nodules, and bilateral pleural effusion (Fig. 3B). One other patient showed right paratracheal lymphadenopathy. In our study, there was no evidence of calcification, mass, cavitation, or septal thickenings in any of the patients. The thin-section CT findings of the 12 patients are summarized in Table 3.
In two patients, follow-up thin section CT images acquired one week after the start of antiviral therapy showed complete resolution or a remarkable decrease in size and opacity of the previously observed GGOs and nodules (Fig. 2C).
With increasing concern over the spread of the pandemic H1N1 2009 influenza virus infection worldwide, detailed knowledge of both its clinical and radiologic features can assist the clinical practice and decision-making of physicians facing this emerging pandemic infectious disease. In addition, preparation for this pandemic infectious disease among institutionalized populations in semi-closed settings such as schools, dormitories, military barracks, or prisons should be made since this infection can spread very rapidly and may quickly become unmanageable without prompt and proper management in the early stages of disease spread. In this study, we attempt to describe one incidence of a military outbreak of the pandemic H1N1 2009 influenza virus with a focus on the clinical and radiological features of these patients, as well as the outcome of treatment.
We found common clinical features for all patients with the pandemic H1N1 2009 influenza virus infection in the present study, which included a high fever with accompanying symptoms of cough, rhinorrhea, sore throat, myalgia, or gastrointestinal symptoms such as vomiting or diarrhea. These findings are similar to the symptoms of other influenzas (8) and those found in previous studies that dealt with unspecified populations (3, 5, 9-13).
Generally, laboratory tests, with the exception of serology and culture examinations, are not useful for the specific diagnosis of influenza. Leukocyte counts are variable according to stage; frequently low during the early stages of illness, and later become normal or slightly elevated (15). In the present series, there were no patients with elevated WBC counts and 67% of patients showed lymphocytopenia. These findings are consistent with those found in previous studies that dealt with unspecified populations (3, 5). One additional diagnostic laboratory feature we were able to find was an elevated CRP level while both the ESR and WBC counts remained within a normal range in all patients in our study with pandemic H1N1 2009 influenza virus infection. We believe that elevated CRP levels, combined with a lack of leukocytosis or elevated ESR, may be characteristic of patients with the pandemic H1N1 2009 influenza virus infection, although these laboratory tests might generally not be able to render a specific diagnosis of this influenza viral infection (14). Further detailed comparative studies with a larger number of patients would be required to fully explore this issue.
Most patients in our study did not show a severe hospital course, although one patient's symptoms aggravated into ARDS and needed mechanical ventilation before laboratory tests revealed that he had the pandemic H1N1 2009 influenza virus infection. We considered the possibility that co-infection of the pandemic H1N1 2009 influenza virus with other unknown respiratory pathogens under the leukocytopenia status could have attributed to this symptom aggravation (9, 10), particularly as he was a soldier serving in a different unit from the majority of other patients. In addition, the symptom aggravation had occurred after he had been in contact with a patient infected with the pandemic H1N1 2009 influenza virus and there were no pandemic H1N1 2009 influenza virus cases in the unit the patient had been enrolled in. However, since his respiratory symptoms and high fever did not respond to other previous antibiotic treatment, there is still the possibility that the disease course in this case could have been solely due to a severe manifestation of the pandemic H1N1 2009 influenza virus infection.
In the present study, chest radiographs were abnormal in two of 18 patients with H1N1, which was less frequent than that of a previous report, which showed a 42% rate of chest radiograph abnormality (12). This suggests that negative findings on chest radiographs cannot rule out the possibility of the pandemic H1N1 2009 influenza virus infection, particularly in its early stage or for an uncomplicated influenza infection (14). Thin-section CT examination results revealed that abnormal findings were found in six out of 12 patients (50%). The most common CT abnormalities included small GGOs in five out of six patients and nodules in all six patients. However, in the previous studies (12, 13), multifocal consolidations (two of seven patients and 50%) and GGOs (two of seven patients and 25%) were the most common CT findings of the pandemic H1N1 2009 influenza virus infection. The differences between the present study and previous studies could be attributed to the fact that radiological examinations in the present study were performed at the initial presentation, and probably the early stages of the disease; whereas in previous studies (12, 13), radiological examinations were performed at various times after symptom onset.
In our study, there was one patient with bilateral lobar consolidations, which progressed into ARDS. Other investigators (3) also reported similar radiographic features in patients who progressed into ARDS. Although extensive consolidations in the lung might be severe manifestations of primary viral pneumonia from the pandemic H1N1 2009 influenza virus infection, there still remains the possibility of combined pneumonia caused by other pathogens or pulmonary manifestations of ARDS (14).
The clinical courses and outcomes of patients in our study with the pandemic H1N1 2009 influenza virus infection were much better than those of previously reported cases (3, 4). We attribute this to the patient population of our study. All of our patients were healthy young soldiers without any risk factors for complications. Furthermore, according to military rule, any problems concerning health must be notified to the medical department immediately, and thus, our patients may have been detected at an earlier stage of the disease course than would be found for the general population. In comparison with a previous report (3) that showed a high mortality rate in patients with the pandemic H1N1 2009 influenza virus infection, the interval between symptom onset and the emergency department visit (mean 1.7 days in 15 among 18 patients) and symptom onset and the start of antiviral agent therapy (mean 3.9 days) were shorter. In addition, detection and treatment were made more promptly. Therefore, we conclude that an early diagnosis and early antiviral management can improve the clinical course and outcome of the pandemic H1N1 2009 influenza virus infection. However, to understand the detailed relationship between symptom onset to admission or antiviral management and clinical courses or outcomes, further study, including a comparative analysis with a large case series, is warranted.
In our series, most patients (15 out of 18) were from the same barrack, a semi-closed setting; whereas, the three remaining patients from different barracks were thought to be infected from these 15 patients. All patients were healthy young males without any combined chronic disease. Clinical and laboratory results from this homogeneous study population can be helpful in understanding the clinical characteristics of the pandemic H1N1 2009 influenza virus infection in semi-closed settings and in institutionalized populations composed of a young healthy population, such as in schools, dormitories, prisons, or military units. Furthermore, this report demonstrated that a prompt diagnosis and treatment resulted in excellent outcomes and could provide as an example for the detection and management strategy for cluster outbreaks of this virus. However, there may be limitations in applying these results to the larger general population.
In conclusion, in a population of healthy young adults, elevated CRP with normal ESR and WBC levels combined with GGOs and nodules on thin-section CT scans may indicate early signs of infection by the pandemic H1N1 2009 influenza virus.
Figures and Tables
Fig. 1
Clinical course and outcome of all 18 patients.
Figure shows brief clinical course and outcome in 18 patients. One patient (case No. 12) progressed into acute respiratory distress syndrome and was placed on mechanical ventilation. Eventually, patient was taken off mechanical ventilation five days after administration of antiviral agent therapy (oseltamivir phosphate, Tamiflu®). In two patients (case No. 6 and 7), symptoms started to improve before administration of antiviral agent therapy for treatment of pandemic H1N1 2009 influenza virus. RT-PCR = reverse-transcriptase-polymerase-chain-reaction

Fig. 2
Small nodules in 20-year-old man with pandemic H1N1 2009 influenza virus infection.
A. Plain chest radiograph shows small nodular opacity in right upper lung (arrow).
B. Thin-section chest CT shows 10 mm nodule (arrow) in right upper lobe of lung. Several tiny nodules are also shown around nodule.
C. Follow-up thin-section CT obtained one week later indicated that this small nodule decreased in size and opacity (arrow). Several tiny nodules that were present in (B) disappeared.
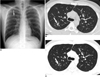
Fig. 3
Consolidations in 22-year-old man with pandemic H1N1 2009 influenza virus infection.
A. Plain chest radiograph shows consolidations in left middle and lower lung. Consolidations are also suspected in right lower lung.
B. Thin-section chest CT image at level of left atrial appendage shows consolidation in left lower lobe (arrow) accompanied by ground-glass opacities in left upper lobe and both lower lobes of lung.
References
1. Influenza A (H1N1) - update 68. World Health Organization. Accessed October 8, 2009. at
http://www.who.int/csr/don/2009_10_02/en/index.html.
2. World now at the start of 2009 influenza pandemic. World Health Organization. Accessed October 8, 2009. at
http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
3. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009. 361:680–689.
4. Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009. 361:674–679.
5. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009. 360:2605–2615.
6. Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med. 2009. 360:2616–2625.
7. Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med. 2009. 360:2667–2668.
8. Interim WHO guidance for the surveillance of human infection with swine influenza A (H1N1) virus. United States Centers for Disease Control and Prevention. 2009. 04. 27. Accessed October 8, 2009. at
http://www.wpro.who.int/NR/rdonlyres/0057E73B-E9DE-470D-8B21-B5E7AFE570D0/0/WHO_guidanceforsurveillanceofswineflu27Apr2009.pdf.
9. Hospitalized patients with novel influenza A (H1N1) virus infection - California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:536.
10. World Health Organization. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Weekly Epidemiological Record. 2009. 84:185.
11. Lee CW, Seo JB, Song JW, Lee HJ, Lee JS, Kim MY, et al. Pulmonary complication of novel influenza A (H1N1) infection: imaging features in two patients. Korean J Radiol. 2009. 10:531–534.
12. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009. 193:1488–1493.
13. Ajlan AM, Quiney B, Nicolaou S, Müller NL. Swine-origin influenza A (H1N1) viral infection: radiographic and CT findings. AJR Am J Roentgenol. 2009. 193:1494–1499.
14. Dolin R. Fauci AS, Braunwald E, Isselbacher KJ, editors. Infections due to DNA and RNA respiratory viruses. Harrison's principles of internal medicine. 2008. 17th ed. New York, USA: McGraw-Hill;1127–1132.
15. Grinblat L, Shulman H, Glickman A, Matukas L, Paul N. Severe acute respiratory syndrome: radiographic review of 40 probable cases in Toronto, Canada. Radiology. 2003. 228:802–809.
16. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008. 246:697–722.
17. Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, et al. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003. 228:395–400.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download