Abstract
Mixed acinar-endocrine carcinoma (MAEC) of the pancreas is extremely uncommon. We report here a rare case of MAEC of the pancreas presenting as watery diarrhea. This is the first report in the English-language literature that describes the imaging findings of MAEC of the pancreas, including computed tomography (CT), magnetic resonance (MR) imaging, and MR cholangiopancreatography features.
Mixed acinar-endocrine carcinoma (MAEC) of the pancreas is extremely rare. However, there have been an increasing number of reports of neoplasms that share many of its characteristic features (1). Because of its rarity, little is known about the clinical features, pathogenesis, and radiological features of MAEC. A diagnosis of MAEC is based on pathologic evaluation, including an analysis of endocrine markers and a histological demonstration of acinar formation (1, 8). To our knowledge, the radiological findings of MAEC have not been reported in the English-language literature. Here, we present CT, MRI and histopathologic findings from a case of MAEC of the pancreas.
A 59-year-old woman presented with watery diarrhea that had persisted for two years. She was referred to our institution with a clinical diagnosis of a vasoactive intestinal peptide-producing tumor (VIPoma) based on her laboratory results. In addition, she had been treated for diabetes mellitus for five years. A laboratory investigation showed increased levels of serum VIP (603 pg/ml; normal range < 20 pg/ml) and serum alkaline phosphatase (162 IU/l; normal range 40-120 IU/l). An arterial blood gas analysis showed metabolic acidosis with hypokalemia.
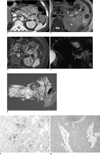
On the CT scans, the tail of the pancreas, covering approximately half of the longest pancreatic axis, was replaced by a heterogeneously hypoattenuating mass-like lesion which enhanced heterogeneously and had a peripheral enhancing capsule-like structure. An approximately 1.5-cm hypervascular nodule was visible in the mass during the hepatic arterial phase (Fig. 1A). No peripancreatic infiltration or direct invasion of neighboring organs was observed. The main pancreatic duct was not visible, and no lymphadenopathy was observed. For further evaluation, MR imaging and MR cholangiopancreatography were performed. The tumor appeared heterogeneously hyperintense on T2-weighted MR images, except for the heterogeneously hypointense nodule located within the mass (Fig. 1B). On contrast-enhanced T1-weighted MR images during the hepatic arterial phase and portal venous phase, the tumor showed heterogeneous enhancement with multiple well or poorly enhancing areas relative to the normal pancreas (Fig. 1C). The nodule within the mass was also strongly enhanced, while the peripheral capsule-like structure was not visible. On MR cholangiopancreatography (TR/TE = 2,050/938), the tumor appeared hyperintense with multiple hypointense nodular lesions (Fig. 1D). An endoscopic ultrasonography revealed a well-defined, homogeneous hypoechoic mass with small cystic lesions in the pancreas tail. A PET-CT revealed that the mass showed signs of hypermetabolic activity.
Diagnosis of the mass based on radiological data indicated a possible diffuse pancreatic ductal adenocarcinoma due to a diffuse heterogeneous mass with an enhancing peripheral capsule-like structure. An endocrine tumor was also considered, due to the presence of an enhancing hypervascular nodule, high signal intensity on T2-weighted MR images, as well as clinical symptoms related to VIP secretion and an elevated VIP level.
The patient underwent a partial pancreatectomy along with a splenectomy. Grossly, the resected tumor measured 8×2.5 cm, and was a well-demarcated, lobulated, soft mass with septation in the pancreatic tail (Fig. 1E). Also present were areas of hemorrhage and focal necrosis with cystic change throughout the tumor. A lymphovascular invasion was observed without invasion into the spleen. An immunohistochemical analysis indicated that the tumor cells were immunoreactive for anti-trypsin (Fig. 1F) and had Periodic acid-Schiff (PAS)-positive diastase-resistant cytoplasmic granules, which was consistent with acinar differentiation. Synaptophysin staining of tumor cells revealed the positivity supporting endocrine nature of this tumor (Fig. 1G). Most tumor cells showed immunohistochemically bidirectional differentiation (i.e., presence of endocrine and acinar cell characteristics, simultaneously). The tumor had morphologic and immunohistochemical features of both acinar cell carcinoma and endocrine tumors. Therefore, the final histopathological diagnosis was a MAEC.
The pancreas is composed of acinar, ductal, and endocrine cells that are morphologically and functionally distinct. Pancreatic tumors usually originate from one of these cell types, most often from ductal cells (2). However, it has been reported that pancreatic neoplasms can exhibit more than one line of cellular differentiation (3). Mixed exocrine-endocrine neoplasms of the pancreas are rare tumors, characterized by the association of an exocrineductal or acinar component and a significant endocrine component that comprises at least one-third to one-half of the total tumor tissue (2, 4). In a study of 645 pancreatic tumors, mixed cell type carcinomas were very rare, representing only 0.2% of all cases (5). MAEC is one of these mixed neoplasms, exhibiting both acinar and endocrine differentiation. Although both acinar cell carcinomas and pancreatic endocrine tumors are distinct entities, their pathological and morphological appearances sometimes mimic each other, and their components can be combined (6). MAEC showed three different combinations: a tumor with separate acinar and endocrine regions identifiable by light microscopy (collision tumor), a mixture of endocrine and acinar cells (intermingled tumor), as well as a tumor with uniform cell population by light microscopy but with amphicrine features, immunohistochemically (2, 8). The histogenesis of mixed exocrine-endocrine tumors of the pancreas is still controversial. The co-existence of exocrine and endocrine elements in these tumors can be attributed to their common embryologic origin (7). Although the biologic aggressiveness of MAEC is still uncertain because of the small number of cases, Klimstra et al. (8) postulated that the behavior of MAEC may be similar to that of acinar cell carcinoma.
To the best of our knowledge, no report focusing on the radiological findings obtained from an MAEC has been published. In the present case, the MAEC diffusely involved the pancreatic tail, which was heterogeneously hypoattenuated compared to the parenchyma of the normal pancreas, with a peripheral capsule-like enhancement observed on CT. These findings are similar to those of diffuse pancreatic ductal adenocarcinoma, although the diffuse morphology of pancreatic ductal adenocarcinoma is an unusual manifestation that represents only approximately 5% of all pancreatic ductal adenocarcinomas (9-11). However, unlike diffuse pancreatic ductal adenocarcinoma, the tumor appeared heterogeneously hyperintense on T2-weighted MR images. In addition, well enhancing portions and a hypervascular nodule were evident on contrast-enhanced T1-weighted MR images, both of which could be seen in characteristic pancreatic endocrine tumors (12, 13). However, some of our findings were uncharacteristic of either pancreatic endocrine tumors or acinar cell carcinomas; namely, diffuse involvement along the long axis of the pancreas rather than a round mass, a hyperintense mass on MR cholangiopancreatography, i.e. heavily T2-weighted MR images (12, 14). The typical radiological findings of acinar cell carcinomas of the pancreas are solitary, exophytic, oval or round, well-demarcated, and hypovascular masses with or without cystic areas (14). The differential diagnosis of a diffuse pancreatic mass also includes diffuse metastasis to the pancreas and pancreatic lymphoma. The presence of a peripheral capsule-like structure, heterogeneous enhancement, including well enhancing areas and a hypervascular nodule, and high signal intensity on MR cholangiopancreatography, are useful for this differential diagnosis (15, 16).
Because MAEC is so rare, its pathogenesis and radiological features remain unclear. Therefore, it is difficult to differentiate MAEC from other pancreatic neoplasms, including acinar cell carcinomas or endocrine neoplasms, by radiological evaluation alone. However, MAEC may be considered when a diffusely involved pancreatic mass shows heterogeneous hypervascular features and hyperintensity on T2-weighted MR images.
In conclusion, we have described the radiological findings of a very rare case of MAEC of the pancreas with diffuse involvement and heterogeneously hypervascular features.
Figures and Tables
Fig. 1
59-year-old woman presented with watery diarrhea that had persisted for two years.
A. On contrast-enhanced axial CT scan during hepatic arterial phase, tail of pancreas, which is approximately half length of longest pancreatic axis, was diffusely replaced by heterogeneously hypo-attenuating mass-like lesion (arrows). Approximately 1.5-cm hypervascular nodule (open arrow) was also visible in mass.
B. T2-weighted axial MR image indicates that mass (arrows) was heterogeneously hyperintense compared to normal pancreas, except for heterogeneously hypointense nodule (open arrow) in mass.
C. Contrast-enhanced T1-weighted axial MR image during hepatic arterial phase showed that tumor (arrows) enhances heterogeneously with multiple well or poorly enhancing areas relative to normal pancreas. Nodule (open arrow) in mass was also found to be well enhanced.
D. MR cholangiopancreatography showed that tumor (arrows) was hyperintense with multiple hypointense nodular lesions.
E. Photograph of surgical specimen reveals well-demarcated, lobulate, soft-tissue mass (arrows) located at tail of pancreas. Areas of hemorrhage and focal necrosis with cystic change in mass are evident. S = spleen
F. Immunohistochemistry results suggest that tumor cells are immunoactive for anti-trypsin (×200).
G. Photomicrograph of mass with immunohistochemical staining showed that tumor cells are immunoactive for synaptophysin (×100).
References
1. Kamisawa T, Tu Y, Egawa N, Ishiwata J, Tsuruta K, Okamoto A, et al. Ductal and acinar differentiation in pancreatic endocrine tumors. Dig Dis Sci. 2002. 47:2254–2261.
2. Klöppel G. Mixed exocrine-endocrine tumors of the pancreas. Semin Diagn Pathol. 2000. 17:104–108.
3. Schron DS, Mendelsohn G. Pancreatic carcinoma with duct, endocrine, and acinar differentiation. A histologic, immunocytochemical, and ultrastructural study. Cancer. 1984. 54:1766–1770.
4. Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004. 445:231–235.
5. Cubilla AL, Fitzgerald PJ. Cancer of the exocrine pancreas: the pathologic aspects. CA Cancer J Clin. 1985. 35:2–18.
6. Yantiss RK, Chang HK, Farraye FA, Compton CC, Odze RD. Prevalence and prognostic significance of acinar cell differentiation in pancreatic endocrine tumors. Am J Surg Pathol. 2002. 26:893–901.
7. Ballas KD, Rafailidis SF, Demertzidis C, Alatsakis MB, Pantzaki A, Sakadamis AK. Mixed exocrine-endocrine tumor of the pancreas. JOP. 2005. 6:449–454.
8. Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994. 18:765–778.
9. Cubilla A, Fitzgerald PJ. Pancreas cancer. I. Duct adenocarcinoma. A clinical-pathologic study of 380 patients. Pathol Annu. 1978. 13:241–389.
10. Clark LR, Jaffe MH, Choyke PL, Grant EG, Zeman RK. Pancreatic imaging. Radiol Clin North Am. 1985. 23:489–501.
11. Choi YJ, Byun JH, Kim JY, Kim MH, Jang SJ, Ha HK, et al. Diffuse pancreatic ductal adenocarcinoma: characteristic imaging features. Eur J Radiol. 2008. 67:321–328.
12. Pamuklar E, Semelka RC. MR imaging of the pancreas. Magn Reson Imaging Clin N Am. 2005. 13:313–330.
13. Choi EK, Park SH, Kim DY, Kim KW, Byun JH, Lee MG, et al. Unusual manifestations of primary pancreatic neoplasia: radiologic-pathologic correlation. J Comput Assist Tomogr. 2006. 30:610–617.
14. Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, et al. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005. 184:511–519.
15. Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000. 174:671–675.
16. Klein KA, Stephens DH, Welch TJ. CT characteristics of metastatic disease of the pancreas. Radiographics. 1998. 18:369–378.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download