This article has been corrected. See "Erratum" in Volume 11 on page 588.
Abstract
Nutcracker syndrome occurs when the left renal vein (LRV) is compressed between the superior mesenteric artery and the aorta, and this syndrome is often characterized by venous hypertension and related pathologies. However, invasive studies such as phlebography and measuring the reno-caval pressure gradient should be performed to identify venous hypertension. Here we present a case of Nutcracker syndrome where the LRV and intra-renal varicosities appeared homogeneously hyperintense on magnetic resonance (MR) fast-spin-echo T2-weighted imaging, which suggested markedly stagnant intravenous blood flow and the presence of venous hypertension. The patient was diagnosed and treated without obtaining the reno-caval pressure gradient. The discomfort of the patient lessened after treatment. Furthermore, on follow-up evaluation, the LRV displayed a signal void, and this was suggestive of a restoration of the normal LRV flow and a decrease in LRV pressure.
Nutcracker phenomenon refers to the compression of the left renal vein (LRV) between the superior mesenteric artery (SMA) and the aorta, and the associated symptoms are defined as Nutcracker syndrome (1). The underlying pathophysiology of Nutcracker syndrome is not yet understood; however, it has been proposed that these related symptoms are caused by venous hypertension in the entrapped LRV (2). Making the diagnosis is based on the assessment of the pathological anatomy and physiology.
We present here the case of a 24-year-old male who presented with intermittent left upper abdominal and flank pain without hematuria, and the patient was diagnosed as having Nutcracker syndrome by using magnetic resonance (MR) fast-spin-echo (FSE) T2-weighted imaging (T2WI). To the best of our knowledge, this is the first report of using MR FSE T2WI to assess a patient with Nutcracker syndrome.
Institutional Review Board approval was obtained for this case report. A 24-year-old man (Body Mass Index = 19.4) suffered from recurrent, intermittent dull upper abdominal pain for more than one year. He had visited several hospitals for help, and the endoscopic study revealed gastritis and the presence of shallow ulcers. Sonography revealed cholelithiasis. Conservative therapy was recommended and the pain lessened over time.
The patient then reported to our emergency room complaining of acute upper abdominal pain and left flank pain with radiation to the left infra-scapular region. He reported having similar attacks over the previous six months. An abdominal computed tomography (CT) scan revealed a heterogeneously enhanced low-attenuation left kidney (Fig. 1A), and acute pyelonephritis was suspected. There were dilated anterior and posterior LRVs (Fig. 1), which drained to a common LRV. No thrombi were noted in the LRVs. A narrow aorto-mesenteric segment of the common LRV was also noted (Fig. 1A), which was overlooked at that time, and it was probably considered to be a normal variant. The patient had no fever, and the urine analysis revealed no evidence of urinary tract infection.
MR cholangiopancreatography was performed due to his persistent upper abdominal and left flank pain and his history of cholelithiasis. Interestingly, the LRV and intrarenal varicosities appeared as bright as the gallbladder and duodenum (Fig. 1B, C) on the MR FSE T2WI (slice thickness = 7.5 mm; TE = 92.88 ms; TR = 7058.82 ms; echo train length = 26; matrix = 256 × 224; flip angle = 90). An interventional radiologist was consulted, and these findings were thought to be due to marked flow stagnation in the LRVs, which inferred the presence of venous hypertension. Non-compensatory Nutcracker syndrome was strongly suspected when considering the anatomic characteristics and also because no extra-renal collateral circulation could be identified (3).
A vascular interventional radiologist and vascular surgeon were consulted. After a thorough discussion with the patient and his family, he decided to undergo LRV reimplantation without stent placement for financial reasons. The LRV was re-implanted 1.5 cm below its original location. Before surgery, the patient had reported an initial visual analogue pain score (VAS) of 9, which fell to two points on the 5th day post-LRV re-implantation.
However, the same pattern of abdominal and flank pain again arose one month after surgery, with a reported VAS score of 10. A repeat CT scan revealed distention of both the anterior and posterior LRVs, and the common LRV in the aorto-mesenteric space remained entrapped. Therefore, the patient was referred for endovascular treatment. A metallic stent (12 mm × 60 mm, Zilver; COOK, Bloomington, IN) was inserted across the entrapped segment of the main LRV. The stent was not dilated after the procedure due to the fear of anastomosis site rupture. After the endoprosthesis was inserted, the discomfort gradually lessened to a final VAS score of 1 at three days post-stent insertion, and the patient was discharged. The VAS score remained constant at 1 for at least three months.
A three-month follow-up MR study revealed no stagnation in the main or anterior LRVs, which were completely black on the MR FSE T2WI (Fig. 1D). However, the posterior LRV remained hyperintense (Fig. 1E), and this was considered to indicate a slow intravenous flow rate. The MR images revealed that the posterior LRV was compressed by the left renal artery (Fig. 1E). However, no further treatment was suggested because the patient experienced only mild upper abdominal discomfort.
Diagnosing Nutcracker phenomenon can be done by anatomic assessment, with finding LRV compression between the aorta and SMA, a ratio between the distended and narrowed portions of the LRV of > 1.5 for the anteroposterior diameter (4) and a sharp, rather than 90° branching angle of the SMA from the aorta (2) (Fig. 1F) all indicating the phenomenon. Clinically identifying Nutcracker syndrome combined with venous hypertension is more complicated. Several anatomic findings can indicate venous hypertension, including a ratio between the distended and narrowed portions of the LRV of > 5.0 for the anteroposterior diameter (69% sensitivity and 89% specificity for Nutcracker syndrome) (5), and identification of peri-renal and peri-ureteral collateral veins. However, anatomic assessment alone is usually inadequate for making a conclusive diagnosis. For example, Takebayashi et al. (3) evaluated 44 patients with hematuria of an unknown origin by performing Doppler sonography, and they correlated their findings with the pressure-gradient measurements. In 23 distended LRVs, 30% (7/23) lacked collateral veins or hypertension and 4% (1/23) had hypertension, but no collateral veins. Therefore, physiologic assessment is essential for making the diagnosis.
The standard (although invasive) method of physiologic assessment is measuring the reno-caval pressure gradient during phlebography. If the pressure gradient is > 3 mmHg, the patient is considered to have venous hypertension (6). A non-invasive alternative is to measure the peak velocities in the distended and narrowed segments of the LRV using Doppler ultrasonography, and then calculating a velocity ratio. If the velocity ratio is > 5.0, then the sensitivity and specificity are 80% and 94%, respectively, for diagnosing Nutcracker syndrome. A combined cutoff value of the anteroposterior diameter and velocity ratios yields a sensitivity of 90% and specificity of 100% (5).
However, quantitative analysis of the venous flow by Doppler sonography has potential pitfalls. It is difficult to obtain a spectral Doppler sampling from an entrapped segment of the LRV in the aortomesenteric space. Furthermore, Doppler sonography is neither accurate nor reproducible when quantifying vessel flow due to the inaccuracy of measuring vessel diameters, their variations with systole and the inappropriate angle of insonation.
Using FSE techniques, Bluemke et al. (7) observed that small veins with slow flow gave very high signaling intensities on T2-weighted images, based on the relatively long T2 of blood. The use of FSE MR phlebography for the forearm and calf veins provided routinely good depiction of the venous anatomy.
For the spin echo (SE) imaging, the protons must be exposed to both 90 and 180 radio frequency pulses to emit a signal. Time-of-flight (TOF) signaling loss can occur when the flowing protons do not remain within a selected slice long enough to be exposed to both frequency pulses. Longer echo times permit blood to exit a section of vessel before generating a signal, while slow flow rates lead to signal generation and generating SE sequences where the blood appears black. If a high signaling intensity in a vessel without flow artifacts is detected on an FSE with long echo times, then the flow should be markedly stagnant. In our case, the LRV signaling intensity using FSE T2WI was as bright as that of the bile in the gallbladder or duodenum.
There is a linear relationship between the MR signaling intensity and the velocity of blood flow. The minimal velocity (v) needed for a given SE pulse sequence in a selected slice to visualize dark blood can be calculated from v = slice thickness/(1/2 TE) (8). In our case, the flow velocity in the LRV should be > 16.1 cm/s (0.75 cm/0.04644 s) to allow visualization of a signaling void in a vessel. Takebayashi et al. (3) defined a LRV flow rate of < 15 cm/s as slow and > 30 cm/min as fast. Therefore, in his follow-up MR FSE T2WI images where most of the LRVs appeared as signaling voids, the flow rates were considered as medium or fast.
The relationships between the pressure gradient and flow velocity have also been studied. When a tube is distally collapsed (comparable to an entrapped renal vein), the pressure gradient reportedly decreases as the flow velocity increases (3). In addition, according to Bernoulli's principle for fluid dynamics, a decrease in fluid speed occurs simultaneously with an increase in pressure and vice-versa. Therefore, the flow rate in a markedly congested and hypertensive LRV should be low. However, in Takebayashi et al.'s study (3), there were five cases where the reno-caval pressure gradient was > 3 mmHg and the measured LRV velocity was variable (low or high). They concluded that one cannot predict the reno-caval pressure gradient by measuring the flow velocity with Doppler sonography. Because of their small number of cases and the potential limitations of their ultrasonographic flow measurements, we still believe that a slow LRV flow rate correlates with an increased pressure gradient. This is perhaps why a large LRV velocity ratio indicates a high probability of Nutcracker syndrome (5), since a higher pressure gradient across the aortomesenteric segment results in a higher measured flow rate and therefore, a larger numerator in the ratio.
Time-of-flight MR angiography in the para-sagittal plane is best for assessing the branching angle of the SMA from the aorta. Though similar with MR imaging, multi-detector rows CT and CT angiography can provide multi-planar anatomic assessment; however, MRI is free of radiation and no contrast medium is needed for TOF MR angiography. In addition, the FSE T2WI holds potential value to visualize the stagnant flow in a hypertensive LRV, and this technique can be applied to both diagnosing Nutcracker syndrome and the follow-up.
In conclusion, a hyperintense LRV on MR FSE T2WI indicated marked flow stagnation, and this inferred the presence of venous hypertension and suggested a diagnosis of Nutcracker syndrome. Therefore, we think the value of MRI for assessing Nutcracker syndrome may not only lie in its ability to depict the anatomic characteristics, but also in providing a non-invasive method of assessing venous hypertension. The LRV signaling intensity from MR FSE T2WI can also be used for follow-up. Accordingly, we advocate that a controlled study is needed to establish the correlation between the LRV signaling intensity and the reno-caval pressure gradient in patients with Nutcracker syndrome and Nutcracker phenomenon. For patients with Nutcracker phenomenon and related symptoms, we recommend using MR imaging, including FSE and TOF MR angiography, before performing invasive studies such as phlebography or measuring the pressure gradient.
Figures and Tables
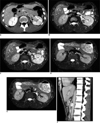
Fig. 1
Nutcracker syndrome in 24-year-old man.
A. Contrast-enhanced CT scan before treatment. 1 = distended anterior left renal vein, 2 = distended posterior left renal vein. Common left renal vein between superior mesenteric artery and aorta is compressed (arrowhead), and left kidney is of relatively lower density than that of right kidney.
B. MR fast-spin-echo T2WI before treatment revealed hyperintense left renal veins (black arrowheads). Note that vascular structures (vena cava, portal vein, superior mesenteric artery, aorta and distal common left renal vein) without flow stagnation appear as signaling voids. There was dark-grey area in anterior left renal vein (white arrowhead), which was flow artifact.
C. MR fast-spin-echo T2WI one slice cranial to A. 1 = anterior left renal vein, 2 = posterior left renal vein, 3 = intra-renal collateral vein. Note that left kidney appears slightly hyperintense compared to that of right kidney, suggesting left renal swelling secondary to venous hypertension.
D. MR fast-spin-echo T2WI performed one month after inserting endoprosthesis. Confluence of anterior and posterior left renal veins is shown (white arrowhead). All visible vascular structures appear as signaling voids. Note that signaling intensities are not different between right and left kidneys.
E. MR fast-spin-echo T2WI one slice cranial to D. Anterior left renal vein gave dark signal. Posterior left renal vein remained bright, and this is probably due to external compression by left renal artery (white arrowhead), which may have caused intravenous flow stagnation.
F. Contrast enhanced CT scan in sagittal plane after left renal vein stent insertion. Note sharp branching angle of superior mesenteric artery from aorta. High density ring in aorto-mesenteric space is cross-section of stent.
References
1. de Schepper A. "Nutcracker" phenomenon of the renal vein and venous pathology of the left kidney. J Belge Radiol. 1972. 55:507–511. [Dutch].
2. Hohenfellner M, Steinbach F, Schultz-Lampel D, Schantzen W, Walter K, Cramer BM, et al. The nutcracker syndrome: new aspects of pathophysiology, diagnosis and treatment. J Urol. 1991. 146:685–688.
3. Takebayashi S, Ueki T, Ikeda N, Fujikawa A. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol. 1999. 172:39–43.
4. Buschi AJ, Harrison RB, Norman A, Brenbridge AG, Williamson BR, Gentry RR, et al. Distended left renal vein: CT/sonographic normal variant. AJR Am J Roentgenol. 1980. 135:339–342.
5. Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996. 198:93–97.
6. Nishimura Y, Fushiki M, Yoshida M, Nakamura K, Imai M, Ono T, et al. Left renal vein hypertension in patients with left renal bleeding of unknown origin. Radiology. 1986. 160:663–667.
7. Bluemke DA, Wolf RL, Tani I, Tachiki S, McVeigh ER, Zerhouni EA. Extremity veins: evaluation with fast-spin-echo MR venography. Radiology. 1997. 204:562–565.
8. Hashemi RH. Hashemi RH, Bradley WG, Lisanti CJ, editors. Flow phenomena. MRI: the basics. 2004. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;293–309.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download