Abstract
Objective
We wanted to determine the clinical feasibility of using non-breath-hold real-time MR-echo imaging for the evaluation of mediastinal and chest wall tumor invasion.
Materials and Methods
MR-echo imaging was prospectively applied to 45 structures in 22 patients who had non-small cell lung cancer when the tumor invasion was indeterminate on CT. The static MR imaging alone, and the static MR imaging combined with MR-echo examinations were analyzed. The surgical and pathological findings were compared with using the Wilcoxon-signed rank test and McNemar's test.
Results
The accuracy, sensitivity and specificity of the combined MR-echo examination and static MR imaging for determining the presence of invasion were 84%, 83% and 85%, respectively, for the first reading session and they were 87%, 83% and 87%, respectively, for the second reading session (there was substantial interobserver agreement, k = 0.74). For the static MR imaging alone, these values were 62%, 83% and 59%, respectively, for the first reader and they were 69%, 67% and 74%, respectively, for the second reader (there was moderate interobserver agreement, k = 0.49). The diagnostic confidence for tumor invasion was also higher for the combined MR-echo examination and static MR imaging than that for the static MR imaging alone (p < 0.05).
Conclusion
The combined reading of a non-breath-hold real-time MR-echo examination and static MR imaging provides higher specificity and diagnostic confidence than those for the static MR imaging reading alone to determine the presence of mediastinal or chest wall tumor invasion when this was indeterminate on CT scanning.
The most important factor affecting the technical feasibility of performing radical resection in patients with lung cancer is the preoperative assessment of the primary tumor. Patients with lung cancer that has invaded the chest wall can achieve prolonged survival when complete resection is performed. The completeness of the resection and the mediastinal nodal status are the most important prognostic factors for patients with chest wall invasion. Whether to do an extrapleural excision or en bloc resection, including the chest wall and ribs, has been controversial (1). Lesions with minimal superficial parietal pleural involvement can be resected by an extrapleural approach without concomitant rib resection as long as clear margins can be obtained (2). Other researchers have argued that the true depth of invasion cannot be accurately determined and that routine en bloc resection for the underlying ribs and fascia results in higher rates of complete, and therefore curative, resections (3). However, such a resection also increases the morbidity and operative mortality (4). Therefore, the preoperative assessment of chest wall invasion and determining whether a tumor has invaded the vital mediastinal structures is important for the surgical planning, selecting the appropriate treatment method and assessing the prognosis of patients.
Both computed tomography (CT) and magnetic resonance imaging (MRI) can show the presence of mediastinal and chest wall tumor invasion. The sensitivity and specificity of CT for the assessment of mediastinal invasion range from 40% to 84% and from 57% to 99%, respectively (5-7). In particular, the length and the angle of the contact between the mass and mediastinal structures and the obliteration of the fat plane have all been used as evidence of local invasion. However, the positive predictive value of CT varies according to the criteria that are used, and the CT signs are not sensitive enough to correctly determine the presence and/or extent of mediastinal or chest wall invasion (5-9).
Although MR imaging has been considered to be valuable in assessing the extent of tumor in some patients, its overall accuracy has not been shown to be better than that of CT (6). In an effort to improve accuracy, several advanced MR techniques, including breath-hold or ECG triggered 3D contrast-enhanced MR angiography, dynamic MRI and real-time cine imaging, have been applied to the evaluations of pleural mesothelioma, radiotherapy planning, the detection of pulmonary embolism and cardiovascular or chest wall invasion (10-16). It has been reported that the sliding motion sign during cine imaging improves the accuracy of the preoperative staging for predicting cardiovascular invasion of a thoracic mass (14, 15). However, this ECG-gated breath-hold cine technique requires repeated breath-holds and it is a difficult examination for old age patients or patients with abnormal pulmonary function.
Non-breath-hold real-time MR-echo imaging using the fast imaging employing steady-state acquisition (FIESTA, GE Healthcare) sequence with a high fidelity driver (HFD) gradient system has recently been introduced for real-time cardiac MR imaging without gating or breath-holding. We think that using this technique without breath-holding it might enable us to see real-time simultaneous movements of the cardiovascular structures and respiration.
The purpose of this study was to determine the clinical feasibility of performing real-time MR-echo imaging that does not require patients to hold their breath for evaluating invasion of non-small cell lung cancer to the cardiovascular structures and the chest wall based on the presence or absence of the sliding motion sign.
Our Institutional Review Board approved this study and all the patients provided us with their written informed consent. The patients' ages ranged from 30-80 years (15 men and 7 women; mean age = 60.4 years).
From January 2006 to December 2006, 91 patients suspected of having non-small cell lung cancer on their chest CT were staged as surgical candidates. Among them, 26 patients with equivocal invasion of the adjacent cardiovascular structures or the chest wall, as based on the CT criteria, were recommended for chest MR imaging with using a real-time MR-echo examination and the conventional static MR imaging. Four patients who underwent MR-echo examinations were excluded because they had small cell lung cancer (n = 2) or distant metastasis (n = 2). Finally, 22 patients underwent both surgery and MR examination. All the 22 patients underwent surgery within a week (mean 3.1 days) of their MR-echo examination.
The CT criteria used for enrollment in this study were based on the obliteration of the fat plane between the thoracic mass and the adjacent cardiovascular structures, or they were based on the extent and angle of contact between the mass and the mediastinal structures or chest wall, which ranged from 3 cm to 5 cm and from 90° to 180°, respectively. The patients were excluded from the study if they had one or more of the following CT features: metastasis, nodular pericardial thickening, mass formation in the chest wall, rib destruction and intraluminal mass formation, or irregular indentation of the adjacent cardiovascular structures.
Four chest radiologists with 4-10 years of experience each independently read the chest CT scans during their routine clinical practice and they selected the cases that were to undergo MR-echo examinations.
The CT scans were performed using 16 or 64-row multidetector scanners (Sensation 16; Siemens Medical Solutions, Forchheim, Germany, or Brilliance 64; Philips Medical Systems, Cleveland, OH) (both with 120 kVp, 100 ref mAs) with contrast administration (3 mL/sec, 100 mL, 370 mg I/mL iopromide [Ultravist 370, Schering]) for a fixed injection duration of 30 seconds, and this was followed by a saline chase for 10 seconds. The CT images were evaluated with using 5-mm slice thickness axial scans.
Static MR imaging for the thorax was performed on a 1.5T system (General Electric EXITE HD, Milwaukee, WI). Before the MR-echo examination, two axial and one coronal static MR examinations were performed with breath-hold. A breath-hold axial T2-weighted single-shot fast spin-echo series (TR 611.3 msec, TE 88.6 msec, slice thickness 7 mm, slice gap 8.4 mm, echo train length 24, number of excitations 2, field of view [FOV] 340 mm, matrix 384 × 192) with using a sensitivity encoding cardiac coil with an eight-coil element and coronal short tau inversion recovery with a body coil (STIR; TR 1400-1500 msec, TE 28.9 msec, TI 120 ms, slice thickness 5.5 mm, slice gap 7 mm, FOV 480 mm, echo train length 14, number of excitations 2, FOV 480 mm, matrix 384 × 192) and axial breath-hold liver acquisition with volume acceleration (3D LAVA; TR 4.1 msec, TE 2.0 msec, slice thickness 5 mm, slice gap 2.5 mm, flip angle 12°, FOV 350 mm, matrix 320 × 192, array spatial sensitivity encoding technique) with contrast enhancement were performed. Gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA, Magnevist; Schering, Berlin, Germany) (0.1 mmol/kgbw, 3 ml/sec) was injected into a cubital vein with an automatic infusion system (Stellant; MedRAD, Pittsburg, PA) and a saline flush (20 ml) was also performed.
A real-time MR-echo examination using an ultra short real-time fast imaging employing steady-state acquisition (FIESTA, GE) pulse sequence (TR 2.4 msec, TE 1.0 msec, flip angle 45°, an array spatial sensitivity encoding technique and a sensitivity encoding cardiac coil with an eight-coil element) was performed after the static MR imaging. This sequence was originally developed for real-time evaluation of the cardiac movement on MRI, which is similar to that for echocardiography.
Initially, a real time 128 × 72 matrix, a 35-36 cm FOV, 66 ms temporal resolution and an 8 mm-section thickness were used to cover the entire mass in the axial, sagittal and coronal planes based on the static MR images. During the real-time MR-echo examinations, the section thickness (5-8 mm) and FOV (27-36 cm) were altered to investigate suspicious invasions or adhesion sites, and the patients were instructed to inspire and expire deeply without breath-holding to determine the presence of sliding motion between the mass and the adjacent cardiovascular structures or the chest wall.
Three chest radiologists (with 5, 2 and 3 years of experience, respectively, in thoracic radiology) who read the chest CT scans as being indeterminate for tumor invasion participated in interpreting the MR imaging. For the analysis of the static MR imaging, two of the radiologists independently analyzed only the static MR image for the presence of invasion and the diagnostic confidence. All the images or files were jointly randomized and shown to the readers in random order. There were two reading sessions for the analysis of the MR-echo examinations. Prospective MR-echo interpretations were done at the completion of the static MR imaging and the real-time examination by moving the axis bar in three orthogonal planes by one chest radiologist (with 5 years experience) by moving the axis bar in three orthogonal planes. This examination took about 5-7 minutes depending on the size of the mass. Another two chest radiologists blindly reviewed the movie files and the static MR images with working in consensus after one month to avoid any recall bias. The gross surgical findings and the pathological findings concerning the adhesion or invasion of mediastinal cardiovascular structures or the chest wall were recorded, and the pathological findings were used as the gold standard to represent the absence or presence of invasion. These results were then reviewed and compared for the presence or absence of the sliding sign on the MR-echo images at the initial examination. A positive sliding sign was interpreted as the absence of invasion or adhesion, and a negative sliding sign was regarded as the presence of adhesion or micro-invasion to the adjacent structures. Therefore, if a mass slid over the pericardium, the myocardium, the great vessels and/or chest wall on the MR echo-images, then it was considered to have no invasion despite that fat plane obliteration was seen on the CT scan.
All the real-time MR-echo examinations were obtained with diagnostic quality without failure. The diagnostic confidence was independently assessed by two radiologists for both the static MR images and the MR-echo examination. The diagnostic confidence was scored as 1: less than 30%, 2: 31-50%, 3: 51-70%, 4: 71-90% and 5: more than 91%.
The sensitivity, specificity, positive predictive value and negative predictive value for the real-time combined MR-echo imaging and the static MR imaging, and the static MR imaging alone were calculated and compared with using McNemar's test. The scored data was analyzed with using the nonparametric Wilcoxon-signed rank test. All the statistical analyses were performed using Medcalc for Windows (Medcalc version 8.0.0.1, Belgium). The interobserver agreement was also calculated and this was considered as slight for a kappa < 0.21, fair for a kappa = 0.21-0.4, moderate for a kappa = 0.41-0.60, substantial for a kappa = 0.61-0.80 or almost perfect for a kappa = 0.80-1.00.
In 22 patients (mediastinal invasion = 12, chest wall invasion = 3, mediastinal and chest wall invasion = 7), 45 structures were in contact with the mass (pericardium = 7, aortic arch = 4, descending aorta = 5, pulmonary artery = 6, superior vena cava = 1, chest wall = 11, trachea = 1, thoracic vertebral body = 2, right atrium = 1, left ventricle = 2, left atrium = 3, diaphragm = 2).
The diagnostic efficacy of the combined MR-echo examination and static MRI and that of the static MRI alone is shown in Table 1. The combined MR-echo examination and static MRI showed a significant increase of specificity in both reading sessions (p < 0.001). The strength of agreement between the two sessions was substantial (k = 0.74) and it was higher than that of the static MR images alone (k = 0.49, moderate). There was no significant difference between the MR echo reading sessions 1 and 2 (p > 0.05). The results of the MR-echo examinations for pathologic invasion and the gross surgical findings are shown in Table 2.
When a sliding sign was present, the mass showed no invasion to the adjacent cardiovascular structures or chest wall (Figs. 1, 2, 3). Although we assessed benign fibrous adhesion or microscopic invasion according to the sliding sign alone during the MR-echo examination, it was interesting that a high signal intensity border was occasionally observed between most of the masses and the cardiovascular structures. This was believed to be due to mediastinal fat or pericardial fluid between the mass and the mediastinal cardiovascular structures (Fig. 1). However, this high signal intensity was not seen for larger masses because of the extrinsic mediastinal structure compression. There was a focal invasion to the diaphragm in the false negative case (a positive sliding sign with pathologic invasion).
The absence of a sliding sign suggested the presence of malignant or benign adhesion between the mass and the mediastinal structures or the chest wall. There were dense fibrous adhesions or invasion between the mass and the adjacent organs on the gross surgical findings. In the false positive cases (a negative sliding sign without invasion), the operator could not dissect between the mass and the adjacent vascular structures or chest wall due to dense fibrous adhesion, and particularly in the pericardium and the superior vena cava. There were no technical problems for the surgery although en bloc resection was performed. Disagreement for the presence of the sliding sign between two sessions was seen at the aortic arch (n = 1), the pulmonary artery (n = 2) and the diaphragm (n = 1).
The diagnostic confidence of the combined MR-echo imaging and the static MR imaging was higher than that of the static MR images alone for both readers (Fig. 4).
The non-breath-hold combined MR-echo evaluation and static MR imaging for assessing mediastinal and chest wall invasion in patients with non-small cell lung cancer showed higher specificity and better diagnostic confidence than that of the static MR imaging alone. Moreover, the interobserver agreement was also higher when the real-time MR-echo examination was performed along with the static MR imaging. Therefore, this technique may increase the ability of physicians to identify the absence of invasion and it enables physicians to better select the operable patients who have a mass that's viewed as indeterminate for mediastinal or chest wall invasion on CT scanning. This technique can also help physicians choose the appropriate surgical treatment, and particularly for those patients who cannot hold their breath during a standard chest MR examination.
The MR-echo technique that uses the fast imaging employing steady-state acquisition (FIESTA, GE) pulse sequence has been developed for cardiac real-time imaging and acquisition, and it is particularly useful for patients with irregular heartbeats and for those patients who are unable to hold their breath. Due to this benefit, MR echo can be used for lung cancer patients who cannot hold their breath efficiently. Moreover, this real-time imaging/evaluation technique can be used to simultaneously determine the presence of chest wall and mediastinal invasion. Unfortunately, MR echo still cannot distinguish benign adhesion that occurs from micro-invasion by a primary tumor, although it produces excellent results that are consistent with the gross surgical findings. Furthermore, false negative sliding signs may be generated by apical tumors and small vessels, and these false negative sliding signs do not show pulsations or movement during breathing.
The 'sliding sign' has been used to determine invasion by a primary mass into adjacent organs on performance of abdominal ultrasonography, and the "sliding sign" can also be applied to thoracic tumors (17, 18). Some authors prefer cine CT or MRI to visualize this sliding sign because of the limitations caused by acoustic shadowing when evaluating tumor invasion to the chest wall for those masses located near vertebra or behind the scapula and shoulder on ultrasonography (14, 15, 19). In addition to chest wall invasion, MR echo is also useful for determining tumor invasion to the descending aorta because the sliding sign should normally be evident between the mass and the descending aorta.
Mediastinal invasion also has been evaluated by cine MRI. Under normal conditions, the pulmonary artery shows protomesosystolic backward and rotational movements, and the other cardiovascular structures also pulsate. Therefore, cine MRI usefully depicts relative movement between the cardiovascular structures and a mass. Seo et al. (15) reported that the sliding sign on breath-hold ECG-gated cine MRI with using a steady state free precession (SSFP) technique was useful for evaluating cardiovascular invasion by a thoracic neoplasm. However, this breath-hold technique is sometimes difficult to perform by patients with a large tumor and dyspnea because it requires multiple sessions to cover the whole mass and three orthogonal planes.
The higher specificity obtained when the static MR imaging is combined with MR-echo examination is particularly valuable when tumor invasion is indeterminate on CT because the patients can be enrolled for surgery by using an objective imaging finding (i.e., a positive sliding sign) prior to thoracotomy, and this may also be helpful for the surgical planning. In the false positive cases (a negative sliding sign without invasion), dissection between the mass and the adjacent vascular structures or chest wall could not be performed by a surgeon due to the dense fibrous adhesions. Therefore, the sliding sign could be used to predict if dense fibrous adhesion or microinvasion of the mass to the adjacent structures is present, and pericardiectomy or chest wall resection should be performed along with mass resection by the surgeon. We can also speculate that atelectasis between a mass and the mediastinal structures may cause difficulties when evaluating mediastinal cardiovascular tumor invasion on CT. Therefore, the non-breath hold real-time MR-echo technique offers an objective means to determine the possibility of performing a surgical treatment before thoracotomy when the CT findings are indeterminate, and this imaging technique can be applied to patients who cannot tolerate multiple breath-hold examinations.
The MR-echo examination produces excellent results because of the presence of pulsation in the cardiovascular structures during the examination. To determine the presence of tumor invasion to the descending aorta by lung cancer, real time respiratory movement of the lung mass along with that of the descending aorta in a coronal or sagittal plane is useful. This real time respiratory movement is also useful for determining the presence of chest wall invasion. However, the determination of a sliding motion at the diaphragm or lung apex was difficult because no significant sliding motion occurs between the lung parenchyma and the diaphragm during respiration.
The present study has some limitations. First, we excluded the patients in whom a CT scan showed direct invasion of the heart, major vessels or chest wall, and no case was unresectable. Thus, our study may be subject to a preselection bias, the same as was noted by similar studies (9, 20). Moreover, some patients with an indeterminate result for mediastinal invasion and who did not undergo surgery because of distant metastasis and small cell lung cancer were excluded. It would have been helpful to have enrolled an equally large group of patients who were thought to have direct mediastinal invasion based on their CT findings and who underwent thoracotomy. However, surgeons do not usually perform thoracotomy when there is obvious infiltration into the mediastinum or extension to the main vessels. Second, a pretest probability bias should be considered for the diagnostic tests of this study and an receiver operating characteristic analysis could not be performed due to the limited data and limited number of subjects. Third, for the evaluation of mediastinal structures and the chest wall, one site invasion may have influenced the observation of invasion at other sites in the same subject. However, inspiratory and expiratory lung movement, and mobile and pulsating mediastinal structures were able to be practically evaluated separately, the same as for other previous studies (11, 15, 16).
In conclusion, a non-breath-hold real-time combined MR-echo examination and static MR imaging showed higher specificity and diagnostic confidence than that for static MR imaging alone to determine the presence of mediastinal or chest wall tumor invasion when this is indeterminate on CT scanning.
Figures and Tables
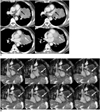 | Fig. 1Positive sliding sign in 54-year-old man with lung cancer.
A. On CT images, fat plane loss and wide contact with left ventricle and atrial appendage (arrows) were observed.
B. Non-breath hold real-time MR-echo image showing sliding sign at left atrial appendage and left ventricle. Note high signal intensity of normal pericardial fluid (white arrows) between mass and left ventricle. No invasion of left atrial appendage or ventricle was observed.
|
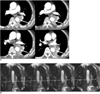 | Fig. 2Positive sliding sign in 73-year-old man with lung cancer.
A. On CT images, mass encased descending aorta over 90 degrees and mass obstructed left lower lobar bronchus (white arrows).
B. Non-breath-hold real-time MR-echo image showing mass sliding upwards (white arrow) over descending aorta on expiration. Left lower lobectomy was performed and no aortic invasion by mass was found during subsequent pathologic examination.
|
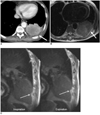 | Fig. 3Positive sliding sign in 71-year-old man with lung cancer.
A, B. On CT (A), and axial fast spin-echo MR (B) images show suspicious fat obliteration and wide contact (arrows) between mass and chest wall in lower lobe.
C. Sagittal MR-echo examination inspiratory and expiratory images show sliding sign or movement over posterior chest wall (white arrows). Surgeon could be confident before operation that mass could be safely removed without en-bloc resection of chest wall. There was no invasion to parietal pleura or chest wall on pathologic examination after operation.
|
 | Fig. 4Diagnostic confidence scores were assessed for two readers. For both readers, diagnostic confidence score was significantly higher for combined MR echo and static MR images than that for static MR images alone (Reader 1 and 2: p < 0.05). |
Table 1
Diagnostic Efficacy of Combined MR-Echo Examination and Static MRI, and Static MRI Alone for Determining Presence of Mediastinum and Chest wall Invasion by Tumor

Table 2
Frequency of Presence or Absence of Sliding Sign by Non-Breath Hold MR-Echo Technique for Mediastinal Structures and Chest Wall

Note.-PC = pericardium, AA = aortic arch, DA = descending aorta, PA = pulmonary artery, SVC = superior vena cava, CW = chest wall, Tr = trachea, VB = vertebral body, RA = right atrium, LA = left atrium, LV = left ventricle, Di = diaphragm, Y = yes, N = no, S1 = first reading session, S2 = second reading session
References
1. Albertucci M, DeMeester TR, Rothberg M, Hagen JA, Santoscoy R, Smyrk TC. Surgery and the management of peripheral lung tumors adherent to the parietal pleura. J Thorac Cardiovasc Surg. 1992. 103:8–12.
2. Downey RJ, Martini N, Rusch VW, Bains MS, Korst RJ, Ginsberg RJ. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg. 1999. 68:188–193.
3. Pairolero PC. Extended resections for lung cancer. How far is too far? Eur J Cardiothorac Surg. 1999. 16:S48–S50.
4. Mansour KA, Thourani VH, Losken A, Reeves JG, Miller JI Jr, Carlson GW, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg. 2002. 73:1720–1725.
5. Glazer HS, Duncan-Meyer J, Aronberg DJ, Moran JF, Levitt RG, Sagel SS. Pleural and chest wall invasion in bronchogenic carcinoma: CT evaluation. Radiology. 1985. 157:191–194.
6. Webb WR, Gatsonis C, Zerhouni EA, Heelan RT, Glazer GM, Francis IR, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology. 1991. 178:705–713.
7. White PG, Adams H, Crane MD, Butchart EG. Preoperative staging of carcinoma of the bronchus: can computed tomographic scanning reliably identify stage III tumours? Thorax. 1994. 49:951–957.
8. Venuta F, Rendina EA, Ciriaco P, Polettini E, Di Biasi C, Gualdi GF, et al. Computed tomography for preoperative assessment of T3 and T4 bronchogenic carcinoma. Eur J Cardiothorac Surg. 1992. 6:238–241.
9. Herman SJ, Winton TL, Weisbrod GL, Towers MJ, Mentzer SJ. Mediastinal invasion by bronchogenic carcinoma: CT signs. Radiology. 1994. 190:841–846.
10. Kauczor HU, Plathow C. Imaging tumour motion for radiotherapy planning using MRI. Cancer Imaging. 2006. 6:S140–S144.
11. Ohno Y, Adachi S, Motoyama A, Kusumoto M, Hatabu H, Sugimura K, et al. Multiphase ECG-triggered 3D contrast-enhanced MR angiography: utility for evaluation of hilar and mediastinal invasion of bronchogenic carcinoma. J Magn Reson Imaging. 2001. 13:215–224.
12. Haage P, Piroth W, Krombach G, Karaagac S, Schaffter T, Gunther RW, et al. Pulmonary embolism: comparison of angiography with spiral computed tomography, magnetic resonance angiography, and real-time magnetic resonance imaging. Am J Respir Crit Care Med. 2003. 167:729–734.
13. Plathow C, Klopp M, Schoebinger M, Thieke C, Fink C, Puderbach M, et al. Monitoring of lung motion in patients with malignant pleural mesothelioma using two-dimensional and three-dimensional dynamic magnetic resonance imaging: comparison with spirometry. Invest Radiol. 2006. 41:443–448.
14. Sakai S, Murayama S, Murakami J, Hashiguchi N, Masuda K. Bronchogenic carcinoma invasion of the chest wall: evaluation with dynamic cine MRI during breathing. J Comput Assist Tomogr. 1997. 21:595–600.
15. Seo JS, Kim YJ, Choi BW, Choe KO. Usefulness of magnetic resonance imaging for evaluation of cardiovascular invasion: evaluation of sliding motion between thoracic mass and adjacent structures on cine MR images. J Magn Reson Imaging. 2005. 22:234–241.
16. Takahashi K, Furuse M, Hanaoka H, Yamada T, Mineta M, Ono H, et al. Pulmonary vein and left atrial invasion by lung cancer: assessment by breath-hold gadolinium-enhanced three-dimensional MR angiography. J Comput Assist Tomogr. 2000. 24:557–561.
17. Lim HK, Kim S, Lim JH, Kim SH, Lee WJ, Chun H, et al. Assessment of pancreatic invasion in patients with advanced gastric carcinoma: usefulness of the sliding sign on sonograms. AJR Am J Roentgenol. 1999. 172:615–618.
18. Lim JH, Ko YT, Lee DH. Sonographic sliding sign in localization of right upper quadrant mass. J Ultrasound Med. 1990. 9:455–459.
19. Kodalli N, Erzen C, Yuksel M. Evaluation of parietal pleural invasion of lung cancers with breathhold inspiration and expiration MRI. Clin Imaging. 1999. 23:227–235.
20. Glazer HS, Kaiser LR, Anderson DJ, Molina PL, Emami B, Roper CL, et al. Indeterminate mediastinal invasion in bronchogenic carcinoma: CT evaluation. Radiology. 1989. 173:37–42.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download