Abstract
Here we report a case in a 41-year-old woman histologically proven cystic embryonal sarcoma of the kidney, with emphasis on the imaging findings and pathological features. A large lobulated solid mass in the cystically dilated pelvocalyceal region was accompanied with hydroureter as depicted on both ultrasound and contrast-enhanced CT images.
A few cases of cystic embryonal sarcoma of the kidney (CESK) with a rapidly fatal outcome have recently been reported. Delahunt and colleagues (1) in 1998 proposed CESK as a new nosologic entity. Two additional cases of CESK were reported in Japan, with an emphasis on the pathological findings (2, 3).
To the best of our knowledge, ours is the first report of a patient with CESK with US and CT findings. Here we report a case of a 41-year-old woman with CESK and review the relevant literature.
A 41-year-old woman presented to the outpatient ward of our hospital with a two-month history of reddish urine and blood clots on voiding and intermittent left flank pain. The medical history revealed that the patient had undergone a medical examination three years prior and results were within the normal ranges.
A physical examination was normal. A urinalysis showed 2+ hematuria (0.5 mg/dl), but no proof of the presence of sugar in the urine or proteinuria. Laboratory findings showed no elevation of the white blood cell count and a normal level of C-reactive protein (CRP).
Initial abdominal ultrasonography (iU 22 unit; Philips Medical Systems, Bothell, WA) demonstrated the presence of a heterogeneous hypoechoic solid mass protruding into the renal pelvis from the upper pole of the left kidney. This was accompanied by hydronephrosis and hydroureter (Fig. 1A). A color Doppler US image showed no vascularity within the mass (Fig. 1B).
Three-dimensional kidney multidetector CT imaging (LightSpeed; GE Healthcare, Milwaukee, WI) was done. On pre-contrast scans, a 5.6 × 5.5 cm hypodense mass with a slightly bulging contour was noted in the left upper polar area. Within the hypodense mass, there were subtle high attenuation areas suggesting hemorrhage or mucin components (Fig. 1C, D). There were also high density lesions in the dilated left proximal ureter. On post-contrast axial scans, a hypodense mass with hydronephrosis and hydroureter was noted in the left kidney (Fig. 1E, F). On reformatted coronal scans, the mass showed an enhancing solid portion with internal reticular densities (Fig. 1G). In the dilated left proximal ureter, there was no definite evidence of an enhancing lesion. A multi-locular cystic and solid tumor of the left upper polar area and extending to the renal pelvis and proximal ureter was the provisional diagnosis. Transitional cell carcinoma could not be ruled out from the pre-operative imaging diagnosis.
We did a left radical nephroureterectomy, and the resected specimen measured 14 × 8 × 6 cm. An 8 × 6 cm myxoid lobulated solid mass was situated in the cystically dilated pelvocalyceal region in the upper pole, which was accompanied by hydroureter (Fig. 1H).
The tumor was seen to have a tan, yellow and brown glistening myxoid lobulated cut surface with multiple scattered hemorrhages. Microscopically, the tumor was mostly composed of primitive-looking or poorly differentiated mesenchymal cells within the loose myxoid and vascular stroma (Fig. 1I). Numerous epithelial-lined cysts and entrapped tubules were scattered throughout the tumor, which was lined by simple or stratified cuboidal to columnar cells with eosinophilic, granular cytoplasm. Mitotic figures were not found in the epithelial cells, but the cells showed a very focal carcinomatous change (Fig. 1J). The tumor also showed extensive areas of recent hemorrhage. Based on immunohistochemical staining, the stromal tumor cells were diffusely reactive for vimentin and non-reactive for desmin and myoglobin. Cyst-lining epithelial cells were positive for vimentin, pan-cytokeratin and epithelial membrane antigen (EMA or MUC1) (Fig. 1K). Ultrastructurally, the stromal tumor cells showed round to ovoid nuclei with regular outlines and an electron-dense rim of heterochromatin.
The final diagnosis was CESK, based on findings of several previous studies found in the literature. The patient was free of disease an eight months after tumor resection.
Cystic embryonal sarcoma of the kidney is an extremely rare renal tumor with a poor prognosis. To the best of our knowledge, only a few cases have been reported in the pathological and urological literature, and there have been no reports about radiological imaging of the tumor.
Recently, the WHO classification of renal neoplasms in children and adults has been revised, but various rare and unclassifiable renal tumors remain. A novel type of malignancy, CESK was reported in 1998 by Delahunt et al. (1), who found 25 similar cases in the files of the National Wilms' Tumor Study Pathology Center. This series of 25 cases was designated as embryonal sarcoma of adult and pediatric kidneys at the 1995 meeting of the United States and Canadian Academy of Pathology.
A review of the English literature revealed that only three cases of cystic embryonal sarcoma have been reported (Table 1). Delahunt et al. (1) proposed CESK as a new nosologic entity in 1998 for a tumor in a 19-year-old Caucasian male who was injured playing rugby (1). CT scans showed a large, well-defined, heterogeneous mass in the right kidney. Following a laparotomy, the mass was found to be hemorrhagic, multilocular and friable. Ito et al. (2) reported another case of CESK with localized submucosal invasion of the renal pelvis in a 12-year-old boy in Japan in 2000. In this case, an imaging study was not done. Following a laparotomy, the lesion was found to be very small, ill defined and mainly localized to the submucosal areas and sinuses of the renal pelvis. Matsumoto et al. (3) in 2002 reported another case of CESK in a 10-year-old boy in Japan. In this patient, abdominal sonography showed the presence of an isoechoic mass that protruded into the renal pelvis from the lower pole of the right kidney. On CT scans, a poorly enhancing mass was noted. MRI revealed that the mass was isointense on T1-weighted images.
Cystic embryonal sarcoma of the kidney has been reported to consist predominantly of undifferentiated malignant mesenchyma and numerous epithelial-lined cysts in myxoid stroma. Delahunt et al. (1) proposed a comprehensive differential diagnosis for CESK. Microscopically, the tubules and cysts of this neoplasm are lined with low cuboidal to flat epithelium composed of cells with spherical nuclei. Vimentin is not expressed by the epithelium in CESK and entrapped glomeruli are not seen. In addition, there is an absence of the typical architectural pattern of haphazardly arranged cells supported by a duplicate fibrovascular stroma, which is seen at least focally in all cases of clear cell sarcoma (1). CESK has different clinical and histological features compared to other renal tumors. CESK appears to be a novel form of a renal malignancy and prognosis is poor if there is early disease recurrence postoperatively or after aggressive chemotherapy. In our patient, we did not administer chemotherapy and the patient was free of disease over an eight-month follow-up period.
The imaging characteristics of CESK have not been well documented because of the rarity of the tumor. In our patient, a poorly enhancing cyst-like mass with hemorrhagic components in the upper polar area that extended to the renal pelvis was found. These findings are similar to findings for mixed epithelial and stromal tumor of the kidney, synovial sarcoma, collecting duct carcinoma and multilocular cystic renal cell carcinoma. Mixed epithelial and stromal tumor of the kidney is a rare benign neoplasm of unknown histogenesis. A similar lesion was previously referred to as cystic hamartoma of the renal pelvis, adult type of mesoblastic nephroma, or cystic nephroma (4). Cystic hamartoma of the renal pelvis forms as an intrarenal multicystic mass adjacent to the pelvocalyceal system, as originally reported by Pawade et al. (5) in 1993. Histopathologically, it is also characterized by a biphasic proliferation of epithelial and mesenchymal elements. Adsay et al. (6) recommended that cystic hamartoma of the renal pelvis and multilocular cystic nephroma should be presented under the name of mixed epithelial and stromal tumor of the kidney and stated that the spindle cells of all mixed epithelial and stromal tumor cells were diffusely and strongly positive for muscle markers such as SMA (smooth muscle actin). Another recent report, one by Jung et al. (7), presented two cases of a mixed epithelial and stromal tumor of the kidney with malignant transformation. The tumor in each case was a similar lesion as cystic renal cell carcinoma. Primary synovial sarcoma of the kidney is also extremely rare but might present with a multiloculated cystic tumor. Collecting duct carcinoma also is included as a differential diagnosis. It is characterized by medullary involvement and an infiltrative growth appearance. Furthermore, a true cystic component with regional lymphadenopathy was a more common finding for collecting duct carcinoma when compared with cortical renal cell carcinoma (8). Multilocular cystic renal cell carcinoma is a distinct subtype of renal cell carcinoma that is distinguished by the presence of a solid mass comprising less than 10% of the volume (9), which consists of a well-circumscribed globular cystic mass demarcated from the kidney and surrounding tissues by a fibrous wall.
In our case, the diagnosis between a cystic embryonal sarcoma and mixed epithelial and stromal tumor of kidney with malignant transformation was debated. Both tumors show mixed components of undifferentiated sarcoma and epithelia-lined cystic components. Histologically, however, the main differential points are the sarcomatous components. Mixed epithelial and stromal tumor of the kidney with sarcomatous transformation doesn't show loose myxoid stromal lobules with occasional condensation of round stromal cells as in CESK. Also, the former consists of highly cellular malignant spindle cells with heterogeneous chondrosarcomatous or rhabdomyosarcomatous components, with ovarian-type stroma, which is not found in CESK.
In conclusion, CESK should be considered in the differential diagnosis for a multilocular cystic renal tumor. Further studies are needed to clarify imaging findings for a cystic embryonal sarcoma.
Figures and Tables
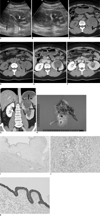
Fig. 1
Imaging findings are presented for 41-year-old woman with cystic embryonal sarcoma.
A. US scan revealed heterogeneous hypoechoic solid mass (black arrow) protruding into renal pelvis from upper pole, which was accompanied by hydronephrosis and hydroureter (white arrows).
B. Color Doppler US image showing no vascularity in mass.
C, D. Precontrast axial scans show hypodense mass with subtle high attenuated portion extending from upper pole to renal pelvis (black arrows).
E, F. Postcontrast axial scans show hypodense mass with slightly enhancing solid portion (black arrows) accompanied by hydronephrosis.
G. Coronal reformatted image shows slightly bulging contour hypodense mass with enhancing solid portion and internal fine reticular densities (black arrows) in left kidney upper pole and extending to renal pelvis.
H. Gross specimen showing 8 × 6 × 4 cm tan, red and yellow myxoid lobulated tumor in markedly dilated pelvocalyceal system at left kidney upper pole.
I. Photomicrograph of specimen showing that myxoid stromal sarcoma grew with protrude lobules that are lined by simple or stratified cuboidal epithelial cells (Hematoxylin & Eosin staining, ×40).
J. Epithelial component showing focal carcinomatous transformation invading renal medulla (Hematoxylin & Eosin staining, ×200).
K. Immunohistochemical staining for epithelial marker cytokeratin revealing strong immunoreactivity on cyst epithelial cells but no immunoexpression on underlying sarcoma component.
References
1. Delahunt B, Beckwith JB, Eble JN, Fraundorfer MR, Sutton TD, Trotter GE. Cystic embryonal sarcoma of kidney: a case report. Cancer. 1998. 82:2427–2433.
2. Ito J, Monobe Y, Sakamoto K, Tanaka H. Embryonal sarcoma of adult and pediatric kidneys: report of a case with localized submucosal invasion of the renal pelvis and long-term survival. Pathol Int. 2000. 50:832–838.
3. Matsumoto H, Inoue R, Tsuchida M, Kawahara N, Hata J, Naito K, et al. Cystic embryonal sarcoma of the kidney. J Urol. 2002. 168:2164–2165.
4. Zhou M, Kort E, Hoekstra P, Westphal M, Magi-Galluzzi C, Sercia L, et al. Adult cystic nephroma and mixed epithelial and stromal tumor of the kidney are the same disease entity: molecular and histologic evidence. Am J Surg Pathol. 2009. 33:72–80.
5. Pawade J, Soosay GN, Delprado W, Parkinson MC, Rode J. Cystic hamartoma of the renal pelvis. Am J Surg Pathol. 1993. 17:1169–1175.
6. Adsay NV, Eble JN, Srigley JR, Jones EC, Grignon DJ. Mixed epithelial and stromal tumor of the kidney. Am J Surg Pathol. 2000. 24:958–970.
7. Jung SJ, Shen SS, Tran T, Jun SY, Truong L, Ayala AG, et al. Mixed epithelial and stromal tumor of kidney with malignant transformation: report of two cases and review of literature. Hum Pathol. 2008. 39:463–468.
8. Pickhardt PJ, Siegel CL, McLarney JK. Collecting duct carcinoma of the kidney: are imaging findings suggestive of the diagnosis? AJR Am J Roentgenol. 2001. 176:627–663.
9. Murad T, Komaiko W, Oyasu R, Bauer K. Multilocular cystic renal cell carcinoma. Am J Clin Pathol. 1991. 95:633–637.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download