Abstract
The increased use of laparoscopic nephrectomy and nephron-sparing surgery has prompted the need for a more detailed radiological evaluation of the renal vascular anatomy. Multidetector CT angiography is a fast and accurate modality for assessing the precise anatomy of the renal vessels. In this pictorial review, we present the multidetector CT angiography appearances of the normal renal vascular anatomy and a spectrum of various anomalies that require accurate vascular depiction before undergoing surgical treatment.
It is important for the surgeons to have extensive preoperative knowledge of the renal vascular anatomy for selecting the proper kidney and for the surgical planning when performing laparoscopic donor nephrectomy (1, 2). Depiction of the vascular variants on the preoperative imaging facilitates the dissection of these vessels and it helps avoid vascular injuries. Planning nephron-sparing surgery in a case of a renal neoplasm also requires precise localization of the renal lesion and its relationship to the renal vasculature (3). Multidetector CT (MDCT) angiography is a fast, reliable and non-invasive modality for the comprehensive evaluation of the renal vasculature (4, 5). MDCT angiography is currently the preferred investigation for evaluating prospective renal donors and it has replaced conventional angiography in many institutions. MDCT angiography can accurately depict the renal arterial and venous anatomy, including the smaller tributaries such as gonadal, lumbar and adrenal veins (4, 5).
Various three-dimensional postprocessing techniques are employed for obtaining angiographic-quality images from the axial CT data. Of the many available reconstruction algorithms, volume rendering and maximum intensity projection (MIP) are most commonly employed (6, 7). The number, size, course and relationships of the renal vessels can be easily demonstrated by using real-time interactive editing of these reconstructions, and the vascular anatomy can be viewed from different perspectives by rotating these images. Curved planar reconstructions are useful to provide a visual summary of the entire vascular anatomy over a single image. As the MIP images lack depth orientation, the volume-rendered images are better than the MIP images for displaying complex anatomy, and especially when overlapping vessels are present (6). The renal arterial anatomy is evaluated on the arterial phase images (1). The major variations of the renal veins are well depicted on the arterial phase images; however, optimal visualization of the large systemic venous tributaries sometime requires additional evaluation of nephrographic phase images (1, 4). All the cases presented in this article were evaluated on a 64 channel multidetector scanner (Brilliance CT, Philips Medical systems, Cleveland, OH) at our institution. The MIP and volume rendered images were reconstructed on a 3 D work station (Extended Brilliance workspace, Philips Medical systems). The purpose of this pictorial review is to describe the usefulness of MDCT angiography for demonstrating the normal and variant renal vascular anatomy. The venous anomalies are described in detail to familiarize radiologists with the various anomalies of the renal veins.
Most individuals have single renal arteries on either side that originate from the abdominal aorta at the level of the L2 vertebra (6) (Fig. 1). The right renal artery has a long downward course due to the inferior position of the right kidney and it lies behind the inferior vena cava (IVC). Classically, each kidney has a single renal vein that usually lies anterior to the renal artery at the renal hilum (Fig. 1). The left renal vein is longer than that of the right renal vein and the left renal vein measures 6-10 cm in length. It normally courses between the superior mesenteric artery and the abdominal aorta and then it joins the medial aspect of the IVC. The right renal vein has a short course, measuring 2 to 4 cm in length, and it joins the lateral aspect of the IVC. Unlike the right renal vein, the left renal vein receives several tributaries, which include an adrenal vein superiorly, a gonadal vein inferiorly and a lumbar vein posteriorly (6) (Fig. 2). Tributaries of the left renal vein, and especially the posterior lumbar branches, are of potential surgical importance if they are noted to be enlarged. The left kidney is preferred for laparoscopic donor nephrectomy due to the longer renal venous pedicle; however, a right nephrectomy may be performed if complex vascular anatomy precludes resection of the donor's left kidney (8).
Variations of the renal veins and IVC are related to the developmental processes in the fetus (9, 10). The renal venous collar is made up laterally by the paired dorsal and ventral primitive renal veins on each side, which are linked to the centrally paired ventral subcardinal and dorsal supracardinal veins, and anastomoses of these four craniocaudally oriented subcardinal-supracardinal veins (Fig. 3). Different anatomic presentations of the renal veins are encountered depending on the persistence or regression of different components of this primitive circumaortic venous network.
More than one artery supplying a kidney is the most common arterial variation, and this is seen in about 24% of cases (11) (Figs. 4, 5). These arteries are divided into two groups: hilar (accessory) and polar (aberrant) arteries. The accessory arteries enter kidney from the hilum along with the main renal artery, whereas the aberrant arteries enter the kidney directly from the capsule outside the hilum. These accessory/aberrant renal arteries usually originate from the abdominal aorta or iliac arteries; however, they can, on rare occasion, arise from the lower thoracic aorta or from the lumbar or mesenteric arteries (6). Early arterial branching or prehilar branching is diagnosed when the first renal branch arises within 1.5 cm of the renal artery ostium (Figs. 5, 6). Early branching is seen in about 12% of the cases (4). Sometimes an aberrant course of the main renal artery may also be observed (a left renal artery arising from the distal abdominal aorta near the bifurcation or a left renal artery arising above the celiac axis; the right renal artery can have a precaval course) (4).
Raman et al. (1, 2) have classified renal vein anomalies into the major or minor subtypes. Major renal vein anomalies are those that result in altered surgical management, including creation of the venous anastomosis in the recipient. The major renal vein anomalies include supernumerary veins and the presence of a late venous confluence. On the left side, the major anomalies also include circumaortic or retroaortic renal veins and a left-sided IVC or a duplicated IVC. The minor venous anomalies bilaterally are those that influence the planning of donor laparoscopic dissection, but they did not alter the venous anastomosis procedure in the recipient and these include anomalies associated with the lumbar, gonadal, adrenal and/or retroperitoneal veins, including the large gonadal and lumbar veins (> 5 mm) and their associated confluence with the main or branch renal veins.
The most commonly encountered venous anomalies are multiple renal veins, which are seen in approximately 15-30% of patients (5, 6). Multiple renal veins are more common on the right side and these occur in up to 30% of individuals (Figs. 8, 9). The other uncommon variants described on the right side include a late venous confluence and minor variants like drainage of a large gonadal vein into the right renal vein. On the right side, a late venous confluence is diagnosed when the renal vein branches coalesce within 1.5 cm of the anastomosis with the IVC (Fig. 10). In one series, late confluence of the venous trunk was reported in about 10% of the right kidneys (2). However, the presence of a late venous confluence on the right side has not been described by other studies as the authors observed that the right renal vein is short, and in almost all cases the confluence occurs within 1.5 cm from the IVC (5). Multiple right renal veins are a contraindication for donor nephrectomy because this variant is associated with a higher incidence of thrombosis of the graft renal vein (4).
The most common anomaly of the left renal venous system is the circumaortic renal vein, and this is seen in 4.4-17% of patients (6, 12). In this variant, the left renal vein bifurcates into the ventral and dorsal limbs that encircle the abdominal aorta (Fig. 11). The appearance of a circumaortic left renal vein depends on the size of the retroaortic venous component, which is variable. Often the retroaortic component joins the IVC at a caudal level (Fig. 12). Sometimes a very small posterior branch from the left renal vein courses posterior to the aorta and the branch drains into the IVC. This posterior branch of the left renal vein is often difficult to differentiate from a lumbar vein or an ascending lumbar vein and in the usual clinical setting, such a small posterior branch is not called a 'circumaortic renal vein' (13).
Other main variants of the left renal vein include a retroaortic left renal vein, double IVC and late confluence of the renal venous trunk. A single retroaortic renal vein is a less common venous anomaly, and this is seen in 1.8-3% of patients (4, 12). A retroaortic left renal vein forms if the dorsal part of the supra-subcardinal vein anastomosis and the intersupracardinal anastomosis persist, whereas the ventral part of the supra-subcardinal anastomosis and the intersubcardinal anastomosis regress. Here, a single left renal vein courses posterior to the aorta and it drains into the lower lumbar portion of the IVC (Fig. 13). Sometimes, the retroaortic renal vein drains into the iliac vein (6). Double IVC is a relatively uncommon condition with a reported incidence of 0.2-3% (9, 14). It results from failure of regression of the embryonic left supracardinal vein. The duplicated left IVC usually drains into the left renal vein, and the left renal vein then crosses anterior to the aorta and joins the right IVC in a normal fashion (Fig. 14). A late venous confluence of the left renal vein is seen in 7-17% of cases (2, 4). On the left side, a late venous confluence is diagnosed when the renal vein branches coalesce within 1.5 cm from the left lateral margin of the aorta (Fig. 15). Preoperative knowledge of the late left venous confluence helps laparoscopic surgeons to anticipate two venous transections if they cannot gain control around the short main renal vein segment. Usually a 5-mm-or-larger gonadal or lumbar vein is present in patients with late venous confluence and it generally it drains into a branch of the main renal vein rather than into the main renal vein itself.
The left renal vein often communicates with the retroperitoneal veins, including the adrenal, lumbar, gonadal and hemiazygous veins. Knowledge of these venous tributaries is relevant and especially the ones that are connected on the posterior aspect of the left renal vein, where the laparoscopic visualization is limited (15, 16). A 5-mm-or-larger gonadal or lumbar vein is seen to drain into the main renal vein in about 40% of cases for the left kidneys or a 5-mm-or-larger gonadal or lumbar vein is seen to drain into a branch renal vein in 14% of the cases for the left kidneys (2) (Figs. 16, 17). In many cases, the gonadal vein is joined by a lumbar branch before eventual insertion into the left renal vein (Fig. 16B). Sometimes an accessory renal vein may drain into the lumbar-gonadal complex (1). Multiple left adrenal veins are seen in 2% of patients and multiple left gonadal veins are seen in 7% of patients (13). Other uncommonly reported venous abnormalities are spontaneous spleno-renal shunts, an isolated renal varix, renal arteriovenous malformations and fistulas (6).
Pre-operative depiction of the complete vascular anatomy is important for the surgical planning, and especially prior to performing laparoscopic nephrectomy and nephron-sparing surgery. Since laparoscopic nephrectomy is performed with a limited field of view, having knowledge of these vascular anomalies reduces the chance of vascular injury and hemorrhage. MDCT angiography is a reliable, non-invasive investigation for evaluating the renal vascular anatomy. The three dimensional volume rendered and MIP techniques are very helpful for accurately displaying various renal vascular anomalies.
Figures and Tables
Fig. 1
Normally seen CT anatomy and relationship of renal vessels in 40-year-old female. Axial maximum intensity projection image shows bilateral renal veins (arrowheads) lying anterior to renal arteries (thin arrows).
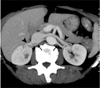
Fig. 2
Depiction of tributaries of left renal vein in 60-year-old male.
A. Axial maximum intensity projection image shows lumbar vein (black arrow) joining at posterior aspect of left renal vein (white arrow).
B. Coronal oblique maximum intensity projection image shows adrenal vein joining superior aspect of left renal vein (white arrow) and gonadal vein joining inferior aspect of left renal vein (black arrowhead).
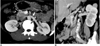
Fig. 3
Schematic diagram showing embryogenesis of inferior vena cava and renal veins.
A. Three pairs of veins (posterior cardinal → subcardinal → supracardinal veins) appear in succession with regression of some portions and persistence of others. Renal collar is formed by intersupracardinal anastomosis dorsally, intersubcardinal anastomosis ventrally and supra-subcardinal anastomosis laterally. Primitive dorsal and ventral renal veins drain into supra-subcardinal anastomoses. Both dorsal renal veins usually regress.
B. After completion of embryogenesis. Right renal vein is formed by ventral limb of primitive right renal vein. Left renal vein develops from intersubcardinal anastomosis, left supra-subcardinal anastomosis and ventral limb of primitive left renal vein. Dorsal intersupracardinal anastomosis regresses.
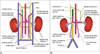
Fig. 4
Multiple equal sized renal arteries on right side in 57-year-old male voluntary kidney donor. Oblique coronal maximum intensity projection image shows three equal size renal arteries supplying right kidney (arrows).

Fig. 5
Accessory renal artery and early branching in 50-year-old female voluntary kidney donor. Anterior volume rendered image shows early branching of main left renal artery (black arrowhead) and presence of accessory renal artery arising from aorta (black arrow). Right inferior phrenic artery is seen arising from right main renal artery (white arrow).
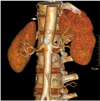
Fig. 6
Early branching and incidentally detected renal artery stenosis in 31-year-old female voluntary kidney donor. Anterior volume rendered image shows early branching of main right renal artery (black arrowhead). Focal renal artery stenosis is also noted in one of segmental branch on left side (white arrow).
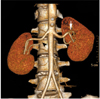
Fig. 7
Incidental renal artery aneurysm in 66-year-old male voluntary kidney donor. Oblique coronal maximum intensity projection image shows small aneurysm in segmental renal artery on right side (arrow).
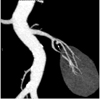
Fig. 8
Supernumerary right renal vein in 30-year-old male voluntary kidney donor. Curved coronal maximum intensity projection (A) and anterior oblique volume rendered (B) images show two right renal veins crossing each other and draining into inferior vena cava (arrows).
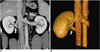
Fig. 9
Multiple supernumerary right renal veins in 27-year-old female voluntary kidney donor. Coronal oblique maximum intensity projection (A) and anterior oblique volume rendered (B) images show four right renal veins (arrows).
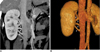
Fig. 10
Late venous confluence of right renal vein in 49-year-old male voluntary kidney donor. Coronal maximum intensity projection image shows late venous confluence of right renal vein (white arrow).
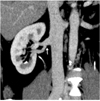
Fig. 11
Classic circumaortic renal vein in 21-year-old female voluntary kidney donor. Axial maximum intensity projection image shows preaortic and retroaortic components (black arrow) of circumaortic left renal vein.
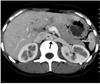
Fig. 12
Circumaortic renal vein in 37-year-old female voluntary kidney donor. Curved coronal maximum intensity projection image shows preaortic (arrowhead) and retroaortic components (white arrow) of circumaortic left renal vein. Retroaortic component joins inferior vena cava at caudal level.
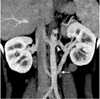
Fig. 13
Single retroaortic renal vein in 52-year-old female who presented with lower end biliary stricture. Axial maximum-intensity-projection (A) and anterior oblique volume rendered (B) images show single left renal vein coursing posterior to aorta (black arrowheads).
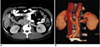
Fig. 14
Double inferior vena cava in 22-year-old male who presented with contracted right kidney. Axial CT (A) and curved coronal maximum intensity projection (B) images show right inferior vena cava (arrows) and left inferior vena cava (arrowheads). Coronal maximum intensity projection image shows left inferior vena cava joining left renal vein.
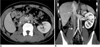
Fig. 15
Late venous confluence of left renal vein in 27-year-old male voluntary kidney donor. Curved coronal maximum intensity projection (A) and anterior oblique volume rendered (B) images show late venous confluence of left renal vein (thick white arrows). Maximum intensity projection image also shows two renal veins on right side (thin arrow). Left adrenal gland and adrenal vein are also nicely seen.
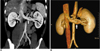
Fig. 16
Large gonadal vein in 44-year-old female who presented with breast carcinoma and liver metastasis. Curved coronal maximum intensity projection (A) and anterior volume rendered (B) images show large gonadal vein (arrows) joining inferior aspect of left renal vein. Volume rendered image shows lumbar vein (arrowhead) joining gonadal vein before finally draining into left renal vein.
References
1. Raman SS, Pojchamarnwiputh S, Muangsomboon K, Schulam PG, Gritsch HA, Lu DS. Utility of 16-MDCT angiography for comprehensive preoperative vascular evaluation of laparoscopic renal donors. AJR Am J Roentgenol. 2006. 186:1630–1638.
2. Raman SS, Pojchamarnwiputh S, Muangsomboon K, Schulam PG, Gritsch HA, Lu DS. Surgically relevant normal and variant renal parenchymal and vascular anatomy in preoperative 16-MDCT evaluation of potential laparoscopic renal donors. AJR Am J Roentgenol. 2007. 188:105–114.
3. Smith PA, Marshall FF, Corl FM, Fishman EK. Planning nephron-sparing renal surgery using 3D helical CT angiography. J Comput Assist Tomogr. 1999. 23:649–654.
4. Holden A, Smith A, Dukes P, Pilmore H, Yasutomi M. Assessment of 100 live potential renal donors for laparoscopic nephrectomy with multi-detector row helical CT. Radiology. 2005. 237:973–980.
5. Chai JW, Lee W, Yin YH, Jae HJ, Chung JW, Kim HH, et al. CT angiography for living kidney donors: accuracy, cause of misinterpretation and prevalence of variation. Korean J Radiol. 2008. 9:333–339.
6. Urban BA, Ratner LE, Fishman EK. Three-dimensional volumerendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics. 2001. 21:373–386.
7. Maher MM, Kalra MK, Sahani DV, Perumpillichira JJ, Rizzo S, Saini S, et al. Techniques, clinical applications and limitations of 3D reconstruction in CT of the abdomen. Korean J Radiol. 2004. 5:55–67.
8. Jacobs SC, Cho E, Dunkin BJ, Flowers JL, Schweitzer E, Cangro C, et al. Laparoscopic live donor nephrectomy: the University of Maryland 3-year experience. J Urol. 2000. 164:1494–1499.
9. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000. 20:639–652.
10. Kandpal H, Sharma R, Gamangatti S, Srivastava DN, Vashisht S. Imaging the inferior vena cava: a road less traveled. Radiographics. 2008. 28:669–689.
11. Ozkan U, Oğuzkurt L, Tercan F, Kızılkılıç O, Koç Z, Koca N. Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol. 2006. 12:183–186.
12. Reed MD, Friedman AC, Nealey P. Anomalies of the left renal vein: analysis of 433 CT scans. J Comput Assist Tomogr. 1982. 6:1124–1126.
13. Kawamoto S, Lawler LP, Fishman EK. Evaluation of the renal venous system on late arterial and venous phase images with MDCT angiography in potential living laparoscopic renal donors. AJR Am J Roentgenol. 2005. 184:539–545.
14. Ng WT, Ng SS. Double inferior vena cava: a report of three cases. Singapore Med J. 2009. 50:E211–E213.
15. Ratner LE, Kavoussi LR, Sroka M, Hiller J, Weber R, Schulam PG, et al. Laparoscopic assisted live donor nephrectomy - a comparison with the open approach. Transplantation. 1997. 63:229–233.
16. Fabrizio MD, Ratner LE, Kavoussi LR. Laparoscopic live donor nephrectomy: pro. Urology. 1999. 53:665–667.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download