Abstract
Objective
To evaluate the clinical and radiological outcomes of transcatheter embolotherapy for treating sporadic pulmonary arteriovenous malformations (PAVMs) that were not associated with hereditary hemorrhagic telangiectasia.
Materials and Methods
Between January 2001 and June 2008, thirty-five sporadic PAVMs were detected in 23 patients. The clinical follow up consisted of assessing the changes of the signs and symptoms of the PAVMs, and radiological evaluation with chest radiographs or chest CT scans.
Results
The lower lung regions (63%) and peripheral locations (86%) were the common locations of the PAVMs. Thirty-four PAVMs (97%) had simple architecture (one arterial feeder within a single pulmonary segment). Technical success was achieved in 33 PAVMs (94%); two cases of technical failure were due to catheterization failure (n = 1) and too large a feeding artery (17 mm) that disabled embolotherapy (n = 1). Coils and Amplatz vascular plugs were used in 30 and three PAVMs, respectively. Inadvertent placement of one coil (n = 1) and pulmonary infarction (n = 1) occurred, but no relevant symptoms developed. For the 13 patients with available data, the mean arterial O2 saturation changed significantly from 92% to 98%. Complete or near-complete involution of the sac was observed in 30 of the 33 embolized PAVMs (91%). In these 33 embolized PAVMs, the mean sac diameter significantly decreased from 17.83 mm to 0.68 mm.
Conclusion
Sporadic PAVMs are mostly the simple type with predominance in the lower lobe and peripheral locations. Transcatheter embolotherapy with coils or Amplatz vascular plugs is a safe and effective treatment for sporadic PAVMs and this provides excellent functional and radiological improvement.
Pulmonary arteriovenous malformations (PAVMs) are abnormal communications between the pulmonary arteries and veins without any intervening capillary beds. Severe complications from PAVMs may occur, including massive hemoptysis and hemothorax, as well as neurologic complications, including transient ischemic attack, cerebral stroke and cerebral abscess due to the right-to-left shunting that facilitates the passage of septic emboli into the cerebral circulation (1). Thus treating PAVMs is justified when this is technically feasible, and even for PAVMs that are asymptomatic. About 70-95% of all PAVMs are associated with hereditary hemorrhagic telangiectasia (HHT or Rendu-Osler-Weber syndrome) (2-7), which is an autosomal dominant genetic disorder that is characterized by recurrent epistaxis, mucocutaneous telangiectasia and visceral vascular involvement, including arteriovenous communications that may develop in virtually any organ and especially in the lungs (2, 3).
The incidence of HHT is particularly high in the American, European and Afro-Caribbean populations (1) and there have been many reports on PAVMs associated with HHT (1, 4-7). The sporadic types of PAVMs, which are not associated with HHT, are rare, and there are only limited reports on their clinical features, radiological characteristics and treatment outcomes. The purpose of this study was to evaluate the clinical and radiological outcomes of performing transcatheter embolotherapy for sporadic PAVMs.
The charts of the subjects of this study were reviewed with the approval of our Institutional Review Board. Informed consent for the chart review was waived. Informed consent was obtained from all the patients at the time of the procedures.
Of a cohort of 25 consecutive patients with PAVMs and who were referred for transcatheter embolotherapy between January 2001 and June 2008, 23 (92%) had sporadic PAVMs and these patients were included in this report; the two excluded patients had PAVMs associated with HHTs. HHT was diagnosed when at least two criteria were met: 1) the patients had cutaneous telangiectasia, epistaxis and/or visceral involvement such as pulmonary, hepatic or central nervous system AVMs, and 2) there was a first degree family history of HHT (8). The 23 included patients consisted of 21 females and two males, and their average age was 48.1 years (range: 12-75 years).
All the patients underwent complete diagnostic pulmonary angiography before their embolotherapy. A 5-Fr Cobra or Headhunter catheter (Cook, Bloomington, IN) was used to access the pulmonary arteries in both lungs. After selective catheterization, the feeding arteries of the PAVMs were embolized, as close to the sac as possible, with using coils or Amplatz vascular plugs (since 2007) of an appropriate size. The embolic coils we used were platinum coils with synthetic fibers (Tornado coils or Nester coils, Cook). The coils were deployed until repeated injections of contrast medium demonstrated the cessation of flow through the PAVMs.
The choice of whether to use coils or Amplatz vascular plugs was made at the discretion of the operator. The diameter of the feeding artery was measured on the diagnostic angiograms to select a coil of suitable size. The coil diameter was overestimated to avoid accidental coil migration. The true diameter of the artery at the site of coil placement was determined from the diameter of the selected catheter as a reference for magnification corrections. After positioning the first coil, the second coil of the same diameter or a smaller diameter was then positioned. The patients with multiple PAVMs in both lungs were sometimes treated in two sessions, depending on the patient's tolerance and the total amount of contrast medium used.
The clinical follow up consisted of assessing the changes of the signs and symptoms of the PAVMs, and radiological evaluation with chest radiographs and/or chest CT scans.
The characteristics of the assessed PAVMs included their number, a lobar location, a central or peripheral location, the angioarchitecture, the diameters of the feeding artery and the aneurysmal sac, the technical success, the complications during embolotherapy or follow up, the change of the arterial oxygen saturation level and the clinical outcome.
Pulmonary arteriovenous malformations that were located in the right upper, right middle and left upper lung lobes were considered to be located in the upper lung zones, and the PAVMs that were located in the right and left lower lobes were considered to be located in the lower lung zones. A peripheral location was defined as the lung parenchyma within 4 cm of the pleural surface and a central location was defined as the more centrally located portion of the lung parenchyma (9). The simple type angioarchitecture consisted of one or more arterial feeders within a single pulmonary segment, whereas the complex type had two or more arterial feeders from more than one pulmonary segment (2, 6). The diffuse type was defined as diffuse involvement of multiple segments or lobes with numerous, variably sized lesions (10). The technical success was defined as the successful embolization for all visible PAVMs with observing no residual PAVM filling on the final angiogram. The arterial oxygen saturation level (SaO2), which is a parameter of the right-to-left shunt fraction, was calculated by conducting arterial blood gas analysis before and after embolization.
The patient outcome was tracked to the most recent radiological follow up. A PAVM was considered to have been successfully treated if complete or near complete involution (i.e., linear scar only) of the aneurismal sac was noted on the follow-up chest CT scans (6). Disappearance of a sac on the chest radiograph was considered acceptable in those patients who had no follow-up CT scan if the sac had been seen on the chest radiograph taken before embolization (6).
Paired Student's t-tests were used to evaluate the statistical significance of the changes of the SaO2 and the aneurismal sac diameter. The SPSS version 14.0 statistical package (SPSS, Chicago, IL) was used for all the statistical analyses, with p values 0.05 indicating statistical significance.
The PAVMs in all 23 patients were diagnosed as a result of incidental imaging findings (n = 6, 26%), respiratory symptoms (n = 15, 65%) or neurologic events (n = 2, 9%) (Table 1). Embolotherapy improved the respiratory symptoms in all 15 patients who had respiratory symptoms. The neurologic manifestations such as brain abscess and thalamic infarction with associated symptoms were improved by surgical drainage and conservative management, respectively, before embolotherapy.
A total of 35 PAVMs were detected on CT scans in 23 patients, with five patients (22%) having two or more PAVMs each. Twenty-two (63%) PAVMs were located in the lower lung zones (11 each in the right and left lower lobes), and 13 (37%) in the upper lung zones (2 each in the right and left upper lobes, respectively, and 9 in the right middle lobe). Thirty (86%) PAVMs had a peripheral location, and five (14%) had a central location. Thirty-four (97%) PAVMs had simple angioarchitecture, and one (3%) had diffuse angioarchitecture.
The mean diameter of the feeding arteries in the 34 simple PAVMs was 5.15 (median, 2.89 mm; range, 1.5-17 mm), with two arteries having of a diameter < 3 mm, 18 arteries had a diameter in the range 3-5 mm, and 14 arteries had a diameter > 5 mm. In these 34 simple PAVMs, the mean diameter of the aneurismal sac was 18.07 (median, 10.98 mm; range, 4-54 mm). The one diffuse PAVM had an aneurismal sac diameter of 40 mm.
Of the 35 PAVMs, 33 (32 simple and one diffuse) were successfully embolized with no residual filling, making the technical success rate 94% (Figs. 1, 2). Twenty-two patients had one session of embolotherapy, while one patient received two sessions of embolotherapy because there were nine PAVMs in both lungs. One to twenty three coils were used for 29 simple PAVMs (average, 5.4 per PAVM), whereas 54 coils were used for the one diffuse type PAVM. Amplatz vascular plugs (6 or 12 mm in diameter) were used for three simple PAVMs, which had feeding artery diameters of 3, 8 and 10 mm (Fig. 2). Each PAVM required only one vascular plug, with complete or near-complete obliteration of the sac observed during follow up. The vascular plug was oversized 30-50% greater than the feeding artery diameter.
The cases of technical failure were caused by failure of catheterization (n = 1) or too large a feeding artery (17 mm) that disabled the embolotherapy (n = 1). The former case showed no change in the sac size on the follow-up CT scans; this patient did not undergo further treatment. The latter case was not embolized due to the high risk of coil migration into the right atrium; this patient underwent right lower lobectomy to remove the PAVM five days later.
During embolotherapy, one coil was inadvertently placed into the right upper pulmonary artery without any symptomatic change in one patient (No. 5 in Table 1). In another patient (No. 11 in Table 1), a follow-up CT scan three weeks after embolotherapy showed a pulmonary infarction distal to the embolized pulmonary artery (Fig. 3). His vague right chest pain subsided within several days, and pleurisy did not develop.
The SaO2 follow-up data recorded 2-24 months after embolotherapy was available for 13 patients. Their mean arterial oxygen saturation changed significantly, from 92% (range: 74.0 to 99.8%) before embolization to 98% (range: 91.7 to 100%) after embolization (p = 0.027). Radiological examinations, which consisted of chest CT scans and radiography in 11 patients and chest radiography only in 12 patients, during an average follow-up period of 18.88 months (range: 1-75 months) showed complete or near-complete involution in 30 of 33 embolized PAVMs (91%). In these 33 embolized PAVMs, the mean diameter of the sac significantly decreased, from 17.83 ± 9.89 mm (range: 4-40 mm) before embolotherapy to 0.68 ± 2.30 mm (range: 0-10 mm) after embolotherapy (p < 0.001). In the remaining three PAVMs, the aneurismal sac diameter decreased, from 36 to 10 mm, from 8 to 6 mm and from 14 to 7 mm, respectively. There was no case of recanalization or sac enlargement during follow up.
Twenty-three of the 25 consecutive patients with PAVMs and who were referred for transcatheter embolotherapy had sporadic PAVMs. In Western countries, PAVMs are highly associated with HHT, which is a common genetic disorder. The reports on HHT and associated PAVMs have been limited in Asian countries. A greater understanding of the characteristics of sporadic PAVMs can help in their management. For example, we found that respiratory symptoms were most common in the patients with sporadic PAVMs, as was the incidental detection of this condition. In contrast, the PAVMs associated with HHT were most commonly detected during systemic screening for HHT (3). In the symptomatic patients with PAVMs associated with HHT, respiratory symptoms are more common than neurologic events, and this is similar to our findings for patients with sporadic PAVMs.
Little is known regarding the radiological characteristics of sporadic PAVMs. We found that multiplicity (22%) was less common, while PAVMs located in the lower lung zone (63%) and peripheral locations (86%) were common. These findings are similar to those of previous reports, which also found that lower lobar (65-91%) and peripheral (73%) locations were common in patients with HHT-associated PAVMs (5, 9, 11), although the incidence of multiplicity in the peripheral locations was much higher (35-65%) (3, 7, 11-13). We also found that the simple type of angioarchitecture was more common in the sporadic PAVMs (97%) than that in the HHT-associated (80-92%) PAVMs (5, 9, 11). Yet the patients with HHT are more likely to have multiple, variably sized lesions, including microscopic lesions (3, 4, 6, 11).
We found that 94% of all the visible PAVMs were successfully embolized with no residual PAVM filling. It is difficult to compare our technical success rate with that of the previous reports because the parameters of technical success in the previous reports was not described in detail and 16-32% of the patients in the previous reports required several sessions of embolotherapy (6, 9). We observed failure of catheterization in one patient who had nine PAVMs, and one of these in the right middle lobe could not be catheterized. The unembolized PAVM did not change in size. Catheterization failure has been reported to be the most common cause of failure during the first embolotherapy attempt, and so these cases required multiple sessions of embolotherapy, and especially in the cases where the feeding arteries were small (11). Another patient had a feeding artery that was too large for embolotherapy (17 mm). In this patient, the large draining vein was too close to the right atrium, so embolotherapy was not attempted. Even with using an occlusion balloon, it would be difficult to occlude a feeding artery with such a large diameter. Surgery is a good alternative in such cases. An Amplatz vascular plug was successfully used in three patients, and each of these three patients had a relatively large feeding artery. This plug can be used for PAVMs with larger arteries (up to 10 mm) and that have high flow, where the alternative may be multiple coils (14, 15).
Complete or near-complete involution was observed in 30 of 33 embolized PAVMs (91%), with a significant decrease of the sac size. This result is comparable to those previously reported results, with complete or near complete involution observed in 75-93% of the PAVMs in 38-155 patients, of whom most (84-96%) had PAVMs associated with HHT (5, 6, 9). In contrast to these previous studies, in which the rate of recanalization was reported to be 2-19%, we observed no case of recanalization of embolized PAVMs (5, 6, 9). The rate of recanalization tends to be higher in complex PAVMs and lower in patients with simple PAVMs (5). The persistence or reperfusion of successfully embolized PAVMs has been reported to occur during recanalization of the embolized vessels, during growth of a missed or previously small accessory artery and when there is collateral flow into the pulmonary artery (6). No case of recanalization or sac enlargement was noted in this study and this could be due to the fact that the majority of the PAVMs were the simple type of PAVMs where single arterial feeders were present, and there were fewer cases of residual PAVMs. We found that the arterial oxygen saturation level significantly increased after embolotherapy. This improvement was related to the improvement of respiratory symptoms in all 15 patients who displayed respiratory symptoms.
Among the observed complications, one patient experienced inadvertent coil embolization (4%), but subsequent pulmonary infarction was not observed. Reflux or migration of the coils within the pulmonary circulation has been reported in 3-6% of patients (5, 16). These coils can migrate to the aneurismal sac or they can reflux into the normal branches of the pulmonary arteries without sequelae. Dislocated coils can be retrieved (5). Three cases of systemic embolization of coils to the mitral valve leaflet or left ventricle have been reported (16, 17). Pulmonary infarction has been reported to occur in 5% of embolotherapy procedures (16). Hemoptysis or chest pain can also occur. Pulmonary infarction may be associated with pleurisy and a high frequency of abnormally enlarged systemic arteries, which can place patients at risk for future hemoptysis (5, 18). Although our patient with pulmonary infarction showed neither pleurisy nor hemoptysis during follow up, these sequelae should be kept in mind when performing further follow-up imaging.
The major limitation of this study was the small number of PAVMs, although the study's patients were collected in a large tertiary referral hospital over an eight year period. No comparisons were made with the PAVMs accompanying HHT because these were much less common during the study period. Another limitation was the retrospective design of the study. In addition, the arterial oxygen saturation measurements and CT scans before and after embolotherapy were not available for all the patients.
In conclusion, our results indicate that sporadic PAVMs are mostly the simple type with predominance in the lower lobe and the periphery of the lung. Transcatheter embolotherapy is a safe and effective treatment for patients with sporadic PAVMs, and this procedure provides excellent functional and radiological improvement.
Figures and Tables
Fig. 1
40-year-old female with large pulmonary arteriovenous malformation in left lower lobe (Patient No. 6). Simple radiograph (A), left selective pulmonary arteriogram (B), and CT angiogram (C) show large 32-mm-diameter pulmonary arteriovenous malformation (arrows) with single arterial feeder (arrowheads in C) and single draining vein. Left pulmonary arteriogram (D) after embolotherapy with placing four Nester coils shows no evidence of pulmonary arteriovenous malformation. Simple radiograph (E) 19 months after embolotherapy shows complete disappearance of pulmonary arteriovenous malformation shadow.
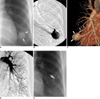
Fig. 2
66-year-old female with incidentally found pulmonary arteriovenous malformation in left lower lobe (Patient No. 13). Left selective pulmonary arteriogram (A) shows 25-mm-diameter pulmonary arteriovenous malformation (arrows) with single arterial feeder (8 mm in diameter) and single draining vein. Spot radiograph (B) shows deployment of 12 mm-diameter Amplatz vascular plug (arrows). Left pulmonary arteriogram (C) after embolotherapy shows no evidence of pulmonary arteriovenous malformation. Follow-up chest radiograph (D) three months later shows near-complete obliteration of previous pulmonary arteriovenous malformation shadow (arrows).
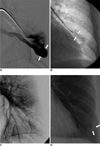
Fig. 3
28-year-old male with small pulmonary arteriovenous malformation in right lower lobe (Patient No. 11). Right pulmonary arteriogram (A) shows small pulmonary arteriovenous malformation (arrows) with single arterial feeder and single draining vein. Right pulmonary arteriogram (B) after embolotherapy with placing two Nester coils shows no evidence of pulmonary arteriovenous malformation. Follow-up CT scan (C) after three weeks shows pulmonary infarction (arrows) distal to embolized pulmonary artery.
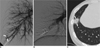
Table 1
Characteristics and Outcomes of Pulmonary Arteriovenous Malformations

Note.-PAVMs = pulmonary arteriovenous malformations, RUL = right upper lobe, RML = right middle lobe, RLL = right lower lobe, LUL = left upper lobe, LLL = left lower lobe, AVP = Amplatz vascular plug, cath. failure = catheterization failure, Sac involution* means complete or near complete involution (i.e. linear scar only) of aneurismal sac, Yes** for treated PAVM(s), NA = not applicable
References
1. Cottin V, Plauchu H, Bayle JY, Barthelet M, Revel D, Cordier JF. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004. 169:994–1000.
2. White RI Jr, Pollak JS, Wirth JA. Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Interv Radiol. 1996. 7:787–804.
3. Cottin V, Chinet T, Lavolé A, Corre R, Marchand E, Reynaud-Gaubert M, et al. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore. 2007. 86:1–17.
4. Gupta P, Mordin C, Curtis J, Hughes JM, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations: effect of embolization on right-to-left shunt, hypoxemia, and exercise tolerance in 66 patients. AJR Am J Roentgenol. 2002. 179:347–355.
5. Mager JJ, Overtoom TT, Blauw H, Lammers JW, Westermann CJ. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol. 2004. 15:451–456.
6. Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI Jr. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2006. 17:35–44.
7. Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999. 74:671–680.
8. Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000. 91:66–67.
9. Remy-Jardin M, Dumont P, Brillet PY, Dupuis P, Duhamel A, Remy J. Pulmonary arteriovenous malformations treated with embolotherapy: helical CT evaluation of long-term effectiveness after 2-21-year follow-up. Radiology. 2006. 239:576–585.
10. Pierucci P, Murphy J, Henderson KJ, Chyun DA, White RI Jr. New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest. 2008. 133:653–661.
11. White RI Jr, Lynch-Nyhan A, Terry P, Buescher PC, Farmlett EJ, Charnas L, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988. 169:663–669.
12. Dines DE, Arms RA, Bernatz PE, Gomes MR. Pulmonary arteriovenous fistulas. Mayo Clinic Proc. 1974. 49:460–465.
13. Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000. 55:959–964.
14. Andersen PE, Kjeldsen AD. Occlusion of pulmonary arteriovenous malformations by use of vascular plug. Acta Radiol. 2007. 48:496–499.
15. Ferro C, Rossi UG, Bovio G, Seitun S, Rossi GA. Percutaneous transcatheter embolization of a large pulmonary arteriovenous fistula with an amplatzer vascular plug. Cardiovasc Intervent Radiol. 2007. 30:328–331.
16. Prasad V, Chan RP, Faughnan ME. Embolotherapy of pulmonary arteriovenous malformations: efficacy of platinum versus stainless steel coils. J Vasc Interv Radiol. 2004. 15:153–160.
17. Haitjema T, ten Berg JM, Overtoom TT, Ernst JM, Westermann CJ. Unusual complications after embolization of a pulmonary arteriovenous malformation. Chest. 1996. 109:1401–1404.
18. Brillet PY, Dumont P, Bouaziz N, Duhamel A, Laurent F, Remy J, et al. Pulmonary arteriovenous malformation treated with embolotherapy: systemic collateral supply at multidetector CT angiography after 2-20-year follow-up. Radiology. 2007. 242:267–276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download