Abstract
Objective
To assess the dynamic activations of the key brain areas associated with the time-course of the sexual arousal evoked by visual sexual stimuli in healthy male subjects.
Materials and Methods
Fourteen right-handed heterosexual male volunteers participated in this study. Alternatively combined rest period and erotic video visual stimulation were used according to the standard block design. In order to illustrate and quantify the spatiotemporal activation patterns of the key brain regions, the activation period was divided into three different stages as the EARLY, MID and LATE stages.
Results
For the group result (p < 0.05), when comparing the MID stage with the EARLY stage, a significant increase of the brain activation was observed in the areas that included the inferior frontal gyrus, the supplementary motor area, the hippocampus, the head of the caudate nucleus, the midbrain, the superior occipital gyrus and the fusiform gyrus. At the same time, when comparing the EARLY stage with the MID stage, the putamen, the globus pallidus, the pons, the thalamus, the hypothalamus, the lingual gyrus and the cuneus yielded significantly increased activations. When comparing the LATE stage with the MID stage, all the above mentioned brain regions showed elevated activations except the hippocampus.
A part from the very limited information available from human subjects with brain lesions, the studies that have been conducted on subjects with epilepsy, the rare studies of electrical stimulation of the brain and animal studies, and especially those using rodents, have been the major source of information about the neural mechanisms that control sexual arousal/behavior (1). Yet the inferences and information from the animal research are not sufficient since human sexual behavior has species-specific characteristics and human sexual arousal is dependent on the complex influences of culture and context (2). It has recently been proposed that human sexual arousal, which is usually triggered by external stimuli or endogenous factors, is a multidimensional experience that's comprised of four closely interrelated and coordinated components: a cognitive component, an emotional component, a motivational component and a physiological component (3). The cognitive component's contributions to sexual arousal are not completely known, but they involve the appraisal and evaluation of the stimulus, categorization of the stimulus as sexual and an affective response (3, 4). The activation of the physiological system that coordinates sexual function in both sexes can be divided into central arousal, peripheral non-genital arousal and genital arousal (5).
Modern neuroimaging techniques allow the in vivo observation of brain activation that is correlated with sensory or cognitive processing and emotional states. The previous studies using positron emission tomography (PET) (3, 4, 6-8) or functional magnetic resonance imaging (fMRI) (9-15) have mostly been focused on visual sexual stimuli such as visual erotica, and these studies have shown increased neural activities in several cerebral regions, including the inferior frontal gyrus, the inferior temporal gyrus, the cingulate gyrus, the insula gyrus, the corpus callosum, the thalamus, the hypothalamus, the amygdala, the caudate nucleus and the globus pallidus. The fMRI measures the changes of the regional cerebral activity through blood oxygenation level dependent (BOLD) signal detection, and this modality has methodological advantages over PET: fMRI is noninvasive and it requires no radiotracer injection as in PET, the fMRI temporal resolution is greater than that of PET, which allows detecting the early response to stimuli, and fMRI can be used not only to study the cerebral responses of a group of subjects, but also to study the responses of individual subjects, which is more difficult to do with PET (14, 16, 17).
Moreover, studies are needed to assess and separate the temporal associations of the central nervous system activity and the peripheral/end organ responses to visual sexual stimulation (10, 18). Therefore, the present study used a 3T fMRI scanner to analyze the dynamic activations of the key brain regions associated with the time-course of the sexual arousal evoked by visual sexual stimulation without any invasive objective and subjective measurements via penile plethysmography. In order to identify and quantify the spatiotemporal activation patterns of the key brain regions, each activation period of our fMRI paradigm was divided into three different stages, that is, the EARLY, MID and LATE stages, and this provided information on the time-course neural activation.
This study was designed to evaluate the time-course information on the brain activation associated with the sexual arousal evoked by visual stimuli in healthy males.
Fourteen male subjects with an average age of 25 years old (range: 22-28 years) participated in this study. The inclusion criteria were being right-handed and exclusively heterosexual. The exclusionary criteria were evidence of any psychiatric and/or sexual disorders, as well as evidence of current pharmacological treatment. The potential participants were interviewed to make sure that they fulfilled the criteria. The local ethics committee approved this study, and the subjects gave their written informed consent. After the completion of the study, the participants were asked to fill out a questionnaire to assess their subjective experiences in terms of 'degrees of attractiveness' and 'sexual arousal' on a 5-point scale.
The fMRI study was carried out according to the standard block design protocol with two rest blocks that each lasted for 1 minute and two activation blocks that each lasted for 3 minutes, and the blocks were arranged in the following order: rest-activation-rest-activation.
During the activation period, erotic video clips were shown with the content of consensual sexual interactions between one man and one woman (petting and vaginal intercourse). This content of the video clips was previously approved by a psychologist and an urologist who both majored in sexual medicine. The visual stimuli were generated on a personal computer and then projected via a liquid crystal display projector onto a screen located inside the MRI scanner room. The same video clips were viewed by the volunteers with the help of a mirror fixed on the head radiofrequency coil in front of the subject's forehead.
The BOLD functional images were acquired on a 3.0T MR scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) by means of the T2* weighted echo planar imaging (EPI) pulse sequence with the following parameters: TR = 3,000 ms, TE = 30 ms, matrix size = 64 × 64, FOV = 220 mm, in-plane voxel size = 3.4 mm × 3.4 mm, flip angle = 90° and slice thickness = 5 mm. A total of 160 functional volumes that consisted of 20 transaxial slices parallel to the 'anterior commissure - posterior commissure' line were acquired.
The functional data preprocessing (19) and statistical analyses (20, 21) were performed using the SPM2 (Statistical Parametric Mapping) software package (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). For each subject, the first two functional brain volumes were discarded to allow for the T1 equilibration effects. In the preprocessing steps, the volumes were motion corrected using the realign and reslice functions (22, 23) and the images were spatially normalized to a standard template in the MNI space (using the EPI.mnc SPM template and this resulted in voxels of 2 × 2 × 2 mm). The normalized images were smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel.
In the statistical analysis, a GLM (general linear model) analysis was performed by dividing the activation period into three one-minute durations as three predictors of interest in the EARLY, MID and LATE stages.
To search for the activated areas that were consistent for the whole group of subjects, a voxel-wise fixed-effect group analysis was performed using one-sample t-tests (p < 0.05). Our homemade program, that is, functional and anatomical labeling of brain activation (FALBA) (24), was used to identify and quantify the activations. The brain activity (%) in this study was defined by the percentage of activated voxels out of a total number of voxels of a given anatomical area and the brain activity was used as the index of activation.
The participating subjects rated the visual sexual stimuli in terms of attractiveness and physical arousal based on a scale ranging from 1 (nil) to 5 (maximal increase). The reported scores (mean ± standard deviation [SD]) were 2.9 ± 0.62 for attractiveness and 3.0 ± 0.88 for sexual arousal (Table 1).
Figure 1 illustrates the group result with the fixed-effect activation patterns (p < 0.05), during the EARLY (Fig. 1A), MID (Fig. 1B) and LATE (Fig. 1C) stages with respect to the rest period, respectively, overlaid on the Colin Holmes 27 (ch2) template of the international consortium for brain mapping (ICBM). Tables 2, 3 and 4 show the summary of the time-course brain activations with significance (p < 0.05), which were extracted from Figure 1: the EARLY (Table 2), MID (Table 3) and LATE stages (Table 4).
Figure 2 compares the brain activations (p < 0.05) during the EARLY, MID and LATE stages with respect to the REST period, respectively. When comparing the MID stage with the EARLY stage, a significant increase of the brain activation was observed in the areas of the inferior frontal gyrus, the supplementary motor area, the hippocampus, the head of the caudate nucleus, the midbrain, the superior occipital gyrus and the fusiform gyrus. At the same time, when comparing the EARLY stage with the MID stage, the putamen, the globus pallidus, the pons, the thalamus, the hypothalamus, the lingual gyrus and the cuneus yielded significantly increased activations. Particularly, the globus pallidus and pons yielded no activity during the MID stage.
When comparing the LATE stage with the MID stage, all the above mentioned ROIs yielded significantly increased activations, except for the hippocampus.
The participating subjects rated the visual sexual stimuli as moderately sexually attractive and physically arousing. When contrasting each stage versus the rest period (Fig. 2), we were able to see the spatiotemporal activation patterns in the key ROIs across the three one-minute durations during the sexual arousal.
Various medical imaging studies on visual sexual arousal have recently been performed to evaluate the brain centers associated with the sexual mechanism and function. However, most of these studies have produced different results and conclusions, and this has created uncertainty in the field (6-15). The main reasons for the differing findings are presumably due to a lack of standardized criteria for what constitutes significant activation over the basal levels and the different methodologies that have been used for inducing arousal and recording it. Hence, it is obvious that it is very difficult at present to give a consensus account of the human brain activation to sexual arousal. Since this study has approached the issue with a different view, we hope our findings have resolved some of the contradictory results.
In this study, visual sexual stimulus of a 3-minute long duration was used in order to activate the complex cerebral mechanism involved in central arousal, peripheral non-genital arousal and genital arousal. An increase in activation was observed from the EARLY stage to the LATE stage in the amygdala, the inferior frontal gyrus, the superior occipital gyrus, the fusiform gyrus, the supplementary motor area, the head of the caudate nucleus and the midbrain regions. The EARLY stage was intended to determine the neural correlates of the early sexual arousal responses (i.e., the neural correlates of the cognitive, emotional and motivational components), the MID stage was intended to identify the brain centers that have an influence on the aspects of the onset of a genital response, e.g., the neural correlates of the perception of penile tumescence, which is a process that occurs with a longer latency, and the LATE stage was intended to illustrate the neural responses that pertain to the state of fully developed sexual arousal, which is associated with a higher level of genital responses (14). Therefore, the increase in activation in the above mentioned regions across the three stages confirms that cognitive and physiological components operate through distinct mechanisms and circuitry, although they are likely to affect each other (25).
Moreover, we were able to find variable activations at the midbrain regions during the period of erotic visual stimulation. The substantia nigra and the surrounding areas are responsible for the production of dopamine, which seems to play a major role in penile erection and sexual arousal (26, 27) and there is substantial evidence that dopamine facilitates male sexual behavior (9, 27). Experiments with animals have also shown that the midbrain structures are involved in erection (28, 29). Therefore, we hope that this result is good evidence for the concordance between men's genital responses and the subjective assessments of arousal.
During the EARLY stage as compared to the other stages, both the thalamus and hypothalamus displayed elevated activation. Yet the activation of the hypothalamus in response to visual sexual stimuli has been an inconsistent finding in human (11). This conflicting result is in fact consistent with the contradictory findings in the animal literature on the relation between sexual cues and the activation of brain regions that have been implicated in sexual behavior (30-32).
The next very interesting structure is the amygdala. The amygdala seems to have a key role in processing the meaning of the ongoing sexual stimulus. If the stimulus is processed as positive, then the amygdala will turn on the cascade of neurobiological events leading to full physical sexual arousal, and if the stimulus is processed as negative, then the amygdala will inhibit or totally block any further physical or emotional arousal (5). In the fMRI studies, there is the possibility that the lack of an amygdalar response is related to susceptibility artifact. Therefore, there is conflict concerning the response of the human amygdala to sexual stimuli, with some studies (6, 10, 13, 15) reporting an activation, while others (3, 8, 12, 14) did not show any amygdalar response. As far as the previous animal studies are concerned, they suggest that different parts of the amygdala are involved in the facilitation of erectile functions (33, 34).
In our study, the activation pattern of the amygdala showed elevated activation during the LATE stage with respect to the other stages. Particularly, no activation was found during the first one-minute period. At this same time period, other studies (9, 11) were unable to confirm amygdala activation during penile erection. Interestingly, amygdala deactivation is related to orgasm (35). Therefore, we have come to the conclusion that the activation of the hypothalamus and amydala reflect not only the physiological arousal, but also cognitive processing of sexual stimuli, such as motivation and desire.
The supplementary motor area is involved in the activation patterns of the insula, and these results are consistent with the findings of other studies (9, 11). Particularly, the insular region lies in the proximity of the secondary somatosensory cortex and the insular region is bidirectionally connected to it; both areas relay visceral and somatosensory perceptions related to the processing of the cognitive content of the incoming sensory stimuli (9, 10). A previous study that used relatively short sexual stimulation periods (21 s long) and still erotic pictures to determine the neural correlates of early sexual arousal responses (the neural correlates of cognitive, emotional and motivational components) has shown that visual sexual stimulation caused activities in the right secondary somatosensory cortex, which is a region that's been implicated in the perception of emotions, and in frontal premotor areas, which have been implicated in motor imagery (14).
Moreover, in a comparative study that used video and still pictures, the hypothalamus, the anterior cingulate gyrus and the insular and secondary somatosensory cortices were found to be activated only by viewing video clips and therefore, the researchers came to the conclusion that the activation of these structures should be related to a more complex and articulated level of sexual response (10).
In conclusion, this study provides valuable information on the spatiotemporal dynamics associated with sexual arousal across the three different stages of the activation of the relevant brain areas by using BOLD-based fMRI. This study may have an important practical impact from the view of its potential clinical application for evaluating the process of sexual arousal as well as sexual dysfunction in men.
Figures and Tables
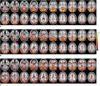 | Fig. 1Regional activation maps (p < 0.05) obtained from group results. Activation contrasts are overlaid over ch2 template: EARLY stage versus REST period (A), MID stage versus REST period (B) and LATE stage versus REST period (C). |
 | Fig. 2Comparison of activations of different key regions of sexual arousal during each stage with respect to rest condition (p < 0.05). |
Table 2
Male Group Results from Fixed-Effect Analysis and Using One-Sample t Tests (threshold significance was set at p < 0.05)

References
1. Levin R, Riley A. The physiology of human sexual function. Psychiatry. 2007. 6:90–94.
2. Schober JM, Pfaff D. The neurophysiology of sexual arousal. Best Pract Res Clin Endocrinol Metab. 2007. 21:445–461.
3. Stoléru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.
4. Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000. 11:162–177.
5. Graziottin A. Sexual arousal: similarities and differences between men and women. J Mens Health Gend. 2004. 1:215–223.
6. Redouté J, Stoléru S, Pugeat M, Costes N, Lavenne F, Le Bars D, et al. Brain processing of visual sexual stimuli in treated and untreated hypogonadal patients. Psychoneuroendocrinology. 2005. 30:461–482.
7. Stoléru S, Redouté J, Costes N, Lavenne F, Bars DL, Dechaud H, et al. Brain processing of visual sexual stimuli in men with hypoactive sexual desire disorder. Psychiatry Res. 2003. 124:67–86.
8. Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, et al. Cerebral activation associated with sexual arousal in response to a pornographic clip: a 15O-H2O PET study in heterosexual men. Neuroimage. 2001. 14:105–117.
9. Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002. 125:1014–1023.
10. Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005. 26:1086–1096.
11. Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B, et al. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage. 2006. 33:689–699.
12. Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.
13. Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002. 16:1–13.
14. Mouras H, Stoléru S, Bittoun J, Glutron D, Pélégrini-Issac M, Paradis AL, et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003. 20:855–869.
15. Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004. 7:411–416.
16. Yang JC, Jeong GW, Lee MS, Kang HK, Eun SJ, Kim YK, et al. Functional MR imaging of psychogenic amnesia: a case report. Korean J Radiol. 2005. 6:196–199.
17. Yang JC. Functional neuroanatomy in depressed patients with sexual dysfunction: blood oxygenation level dependent functional MR imaging. Korean J Radiol. 2004. 5:87–95.
18. Maravilla KR, Yang CC. Sex and the brain: the role of fMRI for assessment of sexual function and response. Int J Impot Res. 2007. 19:25–29.
19. Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalisation of images. Hum Brain Mapp. 1995. 2:165–189.
20. Friston KJ, Holmes AP, Poline J-B, Grasby PJ, Williams SCR, Frackowiak RSJ, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995. 2:45–53.
21. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995. 2:189–210.
22. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996. 35:346–355.
23. Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994. 31:283–291.
24. Lee JM, Jeong GW, Kim HJ, Cho SH, Kang HK, Seo JJ, et al. Qualitative and quantitative measurement of human brain activity using pixel subtraction algorithm. J Korean Radiol Soc. 2004. 51:165–177. [Korean].
25. Janssen E, Everaerd W, Spiering M, Janssen J. Automatic processes and the appraisal of sexual stimuli: toward an information processing model of sexual arousal. J Sex Res. 2000. 37:8–23.
26. Kapp B, Cain M. Smelser N, Baltes P, editors. The neural basis of arousal. The international encyclopedia of social and behavioral sciences. 2001. Oxford: Elsevier Science Ltd;1463–1466.
27. Giuliano F, Allard J. Dopamine and sexual function. Int J Impot Res. 2001. 13:S18–S28.
28. MacLean PD, Ploog DW. Cerebral presentation of penile erection. J Neurophysiol. 1962. 25:29–55.
29. MacLean PD, Denniston RH, Dua S. Further studies on cerebral representation of penile erection: caudal thalamus, midbrain, and pons. J Neurophysiol. 1963. 26:273–293.
30. Michael RP, Clancy AN, Zumpe D. Effects of mating on c-fos expression in the brains of male macaques. Physiol Behav. 1999. 66:591–597.
31. Ferris CF, Snowdon CT, King JA, Sullivan JM Jr, Ziegler TE, Olson DP, et al. Activation of neural pathways associated with sexual arousal in non-human primates. J Magn Reson Imaging. 2004. 19:168–175.
32. Perachio AA, Marr LD, Alexander M. Sexual behavior in male rhesus monkeys elicited by electrical stimulation of preoptic and hypothalamic areas. Brain Res. 1979. 177:127–144.
33. Robinson BW, Mishkin M. Penile erection evoked from forebrain structures in Macaca mulatta. Arch Neurol. 1968. 19:184–198.
34. Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997. 17:5245–5253.
35. Georgiadis JR, Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol. 2005. 493:33–38.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download