Techniques
Preprocedural Imaging
Catheter Embolization
Adjuvant Medications
Embolization Materials
Resorbable materials
Non-Resorbable Materials
Agents for Distal Embolization
* Inert Particles
* Liquids
Agents for Proximal Embolization
* Metallic Coils
* Occluders
* Detachable Balloons
Summary
Complications
Pain
Kidney Failure
Post-Embolization Syndrome
Hematuria
Infections
Accidental Embolization
The Risks Associated with Any Type of Catheterization and the Injection of Contrast Agents
Practical Considerations
Before the Examination
During the Pre-Intervention Check-Up
During the Procedure
Indications
Malignant Tumors: Adenocarcinoma
Pre-Operative Embolization
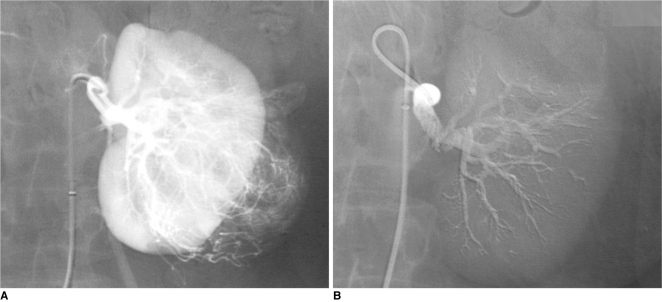 | Fig. 1Preoperative glue embolization of renal cell carcinoma before nephrectomy in 69-year-old man.
A. Selective renal arteriogram: large vascular tumor in lower-pole of left kidney.
B. Control angiogram after embolization of entire renal artery using radiopaque cyanoacrylate/Lipiodol mixture (1:3): complete occlusion of renal artery allows minimal intraoperative blood loss and easier nephrectomy.
|
Palliative Embolization
Pre-Operative Embolization in Patients with Metastases
Benign Tumors: Angiomyolipoma
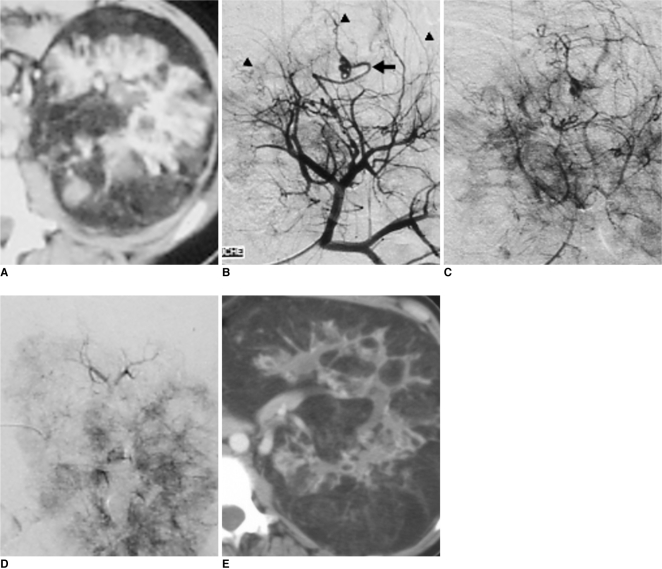 | Fig. 2Recent lumbar pain in 19-year-old patient with Bourneville's tuberous sclerosis.
A-C. CT scan (A) and arteriography (B, C) confirm modifications of renal vascular architecture, as related to fat infiltration and making upper-pole mass opaque (arrowheads) and mass corresponds to hemorrhagic angiomyolipoma. Vascularization of angiomyolipoma is ensured by atypical artery (arrow).
D. Results after superselective embolization with 300-500 µm calibrated microspheres using 3 Fr microcatheter: angiomyolipoma is completely devascularized without infarction of renal parenchyma.
E. CT scan at one year shows evolution of disease with predominant fat infiltration, but no hypervascularization.
|
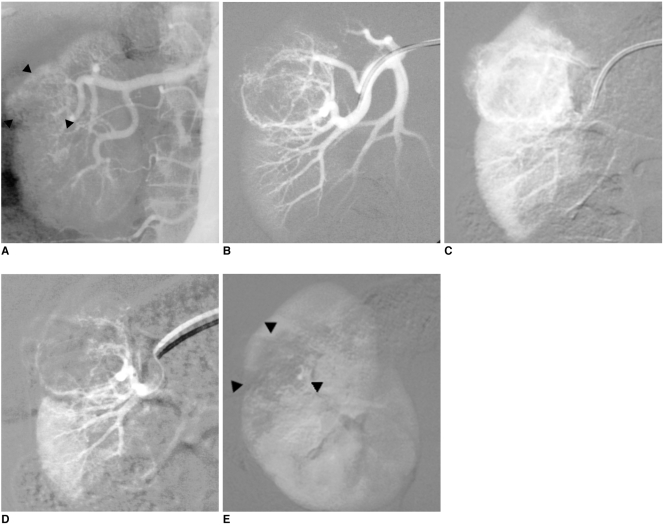 | Fig. 3Preventive embolization of large renal angiomyolipoma in 63-year-old woman.
A. Aortography showing round, well-defined intraparenchymatous mass 4 cm in diameter in upper pole of right kidney (arrowheads).
B, C. Selective renal angiogram confirms hypervascularized character of angiomyolipoma.
D, E. Final angiography after using 300-500 µm microspheres to superselectively embolize two main arterial branches with using 3 Fr microcatheter: devascularization of entire angiomyolipoma (arrowheads) with respect to rest of renal parenchyma.
|
Traumatic or Iatrogenic Lesions
In the Acute Phase
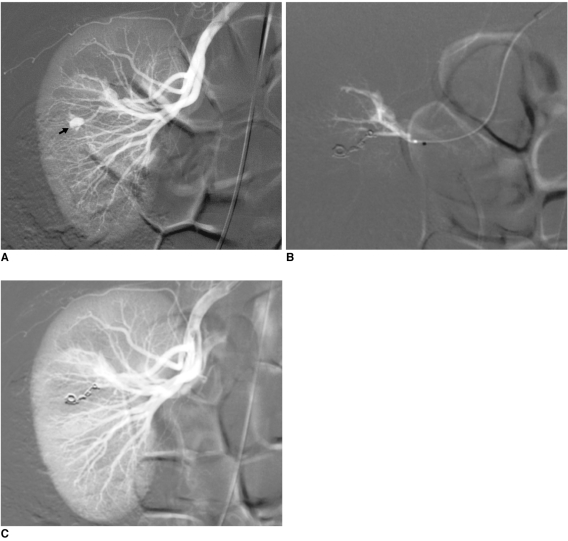 | Fig. 4Emergency embolization for arterial injury after blunt renal trauma in 51-year-old woman.
A. Extravasation of contrast medium (pseudoaneurysm-like lesion) from lower distal-pole branch at selective angiography indicates continuous bleeding (arrow).
B. Selective embolization of feeding artery using detachable microcoils.
C. Control angiogram shows complete and selective occlusion of bleeding branch, with no active bleeding.
|
In the Post-Acute Period
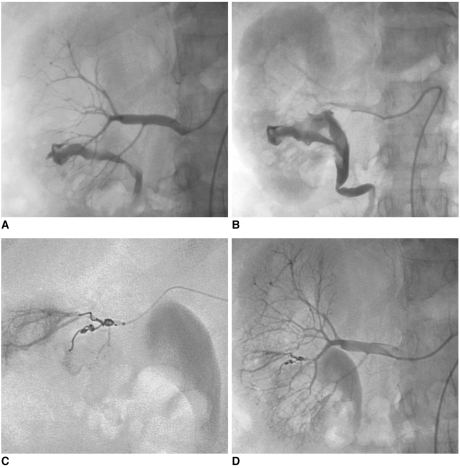 | Fig. 5Massive hematuria with hypovolemic shock two hours after performing percutaneous renal biopsy in 56-year-old woman.
A, B. Presence of high-flow arteriocaliceal fistula on emergency renal angiography with rapid opacification of urinary cavities.
C. Microcoil embolization of two abnormal vessels that were responsible for hematuria.
D. Complete occlusion of fistula and cessation of bleeding are seen on post-embolization angiogram.
|
Delayed Complications
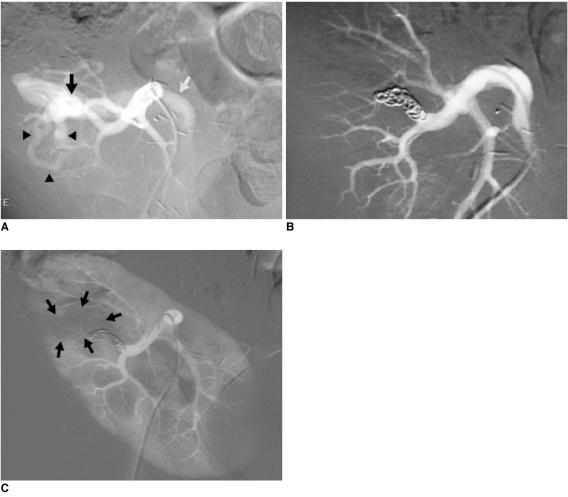 | Fig. 6Progressive arterial hypertension in 43-year-old patient one year after percutaneous renal allograft biopsy.
A. Selective arterial angiogram: there is large arteriovenous fistula in upper-pole segmental branch of transplanted renal artery and pseudoaneurysm (black arrow) with marked arteriovenous shunting (arrowheads) and early venous filling (white arrow). Note absence of nephrogram.
B. Control angiogram after selective embolization of afferent artery with 0.035" coils: complete occlusion of pedicular aneurysm and fistula, and improvement in nephrogram.
C. Post-embolization angiogram (parenchymal phase): renal infarction is seen in less than 10% of renal parenchyma (arrows).
|
Pedicular Aneurysms
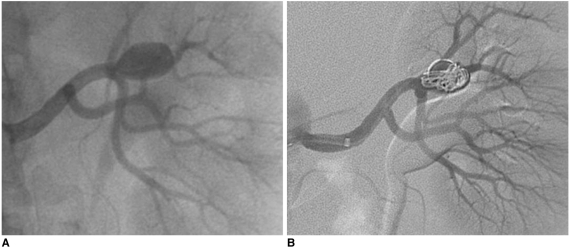 | Fig. 767-year-old man with asymptomatic renal artery aneurysm that was incidentally discovered on CT scan of abdomen.
A. Selective arteriogram demonstrating filling of saccular renal aneurysm arising from hilar branch.
B. Control angiography after embolization of aneurysmal sac across neck with detachable fibered microcoils and using packing technique: there is near complete occlusion of aneurysm and preservation of main renal artery.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download