Abstract
Follicular dendritic cell sarcoma is a rare neoplasm that originates from follicular dendritic cells in lymphoid follicles. This disease usually involves the lymph nodes, and especially the head and neck area. Rarely, extranodal sites may be affected, including tonsil, the oral cavity, liver, spleen and the gastrointestinal tract. We report here on the imaging findings of follicular dendritic cell sarcoma of the abdomen that involved the retroperitoneal lymph nodes and colon. It shows as a well-defined, enhancing homogenous mass with internal necrosis and regional lymphadenopathy.
Follicular dendritic cells are found in primary and secondary lymphoid follicles. These cells participate in the immune system by retaining and presenting antigens for B cells and they also stimulate B cell proliferation and differentiation (1). Follicular dendritic cell sarcoma is a rare neoplasm that arises from these follicular dendritic cells. The disorder was originally described in 1986 in a report on a non-lymphomatous primary lymph node malignancy (2). Several pathological and clinical studies concerned with follicular dendritic cell sarcoma have been reported. However, to the best of our knowledge, follicular dendritic cell sarcoma of the abdomen has not yet been described with a focus on the imaging findings. We report here on two cases of follicular dendritic cell sarcoma of the abdomen that involved the retroperitoneal lymph nodes and the colon, respectively. We also review the relevant medical literature.
A 69-year-old man presented with a 2-month history of intermittent epigastric pain. At the time of presentation, the patient had hypertension that was controlled with medication, and he had no other remarkable past medical history. The physical examination and laboratory tests were unremarkable.
After admission, the patient underwent an upper gastroduodenal endoscopy examination and the result was normal. We then performed ultrasonography (US) and computed tomography (CT) examinations to further evaluate the abdominal pain. Based on the US findings, a large well-defined anechoic mass was identified and it was located in the posterior aspect of the splenic vein. The lesion was seen with posterior acoustic enhancement that suggested the presence of a cystic or necrotic mass (Fig. 1A). Helical CT was performed using a multidetector-row CT (MDCT) scanner (LightSpeed VCT; GE Healthcare; Milwaukee, WI) with 64 detectors. The abdominal CT axial and coronal images (Fig. 1B, C) showed an approximately 5 cm sized, well-defined, necrotic mass with an even peripheral wall in the retroperitoneal area. The mass was located between the left renal vein and the splenic vein and the mass compressed the adjacent pancreas without evidence of direct invasion. There was no calcification detected in the mass. Several abnormal enlarged lymph nodes in the left gastric area were also noted. Based on these imaging findings, metastatic lymph nodes, tuberculosis lymphadenitis and an unusual form of Castleman's disease were considered in the differential diagnosis. The main necrotic mass and abnormal enlarged lymph nodes were removed by surgical excision. On a gross specimen, the mass showed extensive internal necrosis that correlated to the image findings seen on US (Fig. 1D). Spindle to ovoid cells that made up fascicles were identified on a light microscopic examination of the resected mass and lymph nodes. The tumor cells had slightly eosinophilic plump cytoplasm with an indistinct cell border and small nuclei (Fig. 1E). After immunohistochemical staining, the tumor cells showed focal positivity for CD 21 and CD 35. These findings were consistent with a follicular dendritic cell sarcoma.
A 52-year-old woman presented with hematochezia and dyspepsia. The symptoms had developed two weeks prior to the patient's presentation to a local clinic and then colonoscopy was performed at that clinic. According to the colonoscopic findings, an approximate 12 cm intraluminal fungating mass was detected in the descending colon. A gastrointestinal stromal tumor (GIST) in the colon was initially suspected. The patient was transferred to our hospital for further evaluation and management. All of the laboratory findings, including the levels of tumor markers (CA 19-9 and carcinoembryonic antigen), were unremarkable except for a low hemoglobin level (10.5 g/dL). The hemoglobin level was slightly decreased due to hematochezia.
A CT scan (Sensation 64; Siemens Healthcare, Erlangen, Germany) was performed for surgical planning and the multiplanar reconstruction images were obtained. On a pre-enhanced CT scan, the mass showed multiple foci of irregular and dense calcifications in the central portion (Fig. 2A). The axial and coronal post-enhanced CT images (Fig. 2B, C) showed that the mass was well-defined, with a size of 12 cm at the longest diameter. The mass was seen with two homogenously enhancing components and a lobulated margin. The mass was located in the mid-descending colon and the mass was composed of intraluminal and extraluminal components. The intraluminal component showed less enhancement than did the extraluminal component. There was no evidence of adjacent organ invasion as seen the coronal images, but multiple enlarged lymph nodes were noted around the mesocolon and inferior mesenteric vessels. Based on these imaging findings, we suspected that the mass was a malignant GIST or an unusual sarcoma that arose from the descending colon with metastatic lymph nodes for the differential diagnosis. The patient then underwent a left hemicolectomy. During surgery, a well-defined mass that measured 12×10 cm was identified in the mid-descending colon. There was direct focal invasion of the left lateral parietal peritoneum by the mass, but the overall margin of the tumor was well-defined. On a gross specimen, the mass showed a relatively dull yellow color with some portions of necrosis in the intraluminal component, and the non-necrotic extra-luminal component had a pink-tan, solid appearance (Fig. 2D). On a light microscopic examination, the cellular component of the mass was composed of plump spindle cells with open chromatin and small nuclei. These cells had a rather bland appearance and any mitotic figures were not identified. Sclerotic bundles with multifocal calcifications, several lymphocytes, plasma cells and eosinophils were admixed with foamy macrophages. The tumor cells showed focal positivity for CD35 (Fig. 2E). These findings were compatible with a diagnosis of follicular dendritic cell sarcoma.
Follicular dendritic cell sarcoma is a rare neoplasm and it is a neoplastic proliferation of spindle-shaped to ovoid cells that demonstrate the morphological and phenotypical features of follicular dendritic cells. Proliferations of follicular dendritic cells occur in the situation of reactive and neoplastic conditions (3). The neoplastic cells usually demonstrate the phenotype of non-neoplastic follicular dendritic cells. The cells can be positive for one or more markers as determined with immunohistochemical staining, and these markers are also found in non-neoplastic follicular dendritic cells. Among these markers, determining the levels of CD21 and CD35 is helpful for making the diagnosis of follicular dendritic cell sarcoma (4). The diagnosis of follicular dendritic cell sarcoma can be made based on the characteristic ultrastructural or immunohistochemical findings (5).
Clinically, this neoplasm affects both sexes equally. The disease occurs over a wide age range from 14 to 77 years (mean age: 47 years) and this malady typically presents as a painless, slow growing well-defined mass lesion at the involved site (5). The predominant site of tumor involvement in most cases is the lymph nodes. Especially, the disease shows a greater tendency to occur in the head and neck area, yet this disease is also known to occur in extranodal sites such as tonsil, palate, small intestine, mesocolon, pancreas, liver, mesentery, the abdominal wall and the soft tissue of the neck (4, 6-10). Various treatment modalities have been used to treat this tumor, including surgery, chemotherapy and radiation therapy. Complete surgical resection should be considered for localized disease. Good results have been seen for patients who received neoadjuvant radiotherapy and chemotherapy. However, no definite therapeutic approach has demonstrated consistent efficacy (5). In our first case, local recurrence was detected on a follow-up CT scan after eight months from the time of surgery.
Any studies that have focused on demonstrating the imaging findings of follicular dendritic cell sarcoma are limited because of the rarity of this tumor. The presence of this disease has been demonstrated in a few oncological case reports as a large mass with or without regional lymphadenopathy in the abdomen (8, 9). In the radiological literature, Leipsic et al. (11) first described the CT findings of a follicular dendritic cell sarcoma of the mediastinum in 2007. They reported that the tumor was seen as bulky soft tissue mass with internal calcifications on a non-enhanced CT scan.
In our study, one case showed a retroperitoneal mass that represented the nodal type, and the other case demonstrated colon involvement that represented the extranodal type. For the case of the nodal type, the retroperitoneal mass was seen as a well-defined lesion with central necrosis and regional lymphadenopathy. There was no calcification or hemorrhage within the mass. The CT findings were similar to those of metastatic lymphadenopathy, tuberculosis lymphadenitis and an unusual form of Castleman's disease (12). Among these diseases, Castleman's disease has been found in association with a follicular dendritic cell sarcoma, which suggests that it may represent a precursor lesion (13). Thus, it is possible that a follicular dendritic cell sarcoma of the abdomen will share the imaging findings of Castleman's disease in some respects, and especially for the nodal type.
In the case of the extranodal type, the colon mass was seen with coarse internal calcification, and this was unlike the nodal type. The relatively low enhanced portion of the intraluminal component, as seen on CT images, showed several mixtures of necrosis on a pathological examination. Although the tumor was of a large size, the tumor showed homogenous enhancement except for the area of necrosis and it was well-demarcated without CT evidence of invasion to any adjacent structure. The differentiating features may be a large area of even internal necrosis and a well-defined margin in the setting of a large tumor, as compared with other colonic masses such as a malignant GIST. Considering these findings, the extranodal type showed imaging findings that were similar to those of the nodal type. We suggest that a well-defined mass with regional lymphadenopathy and homogenous enhancement with internal necrosis are the CT findings of follicular dendritic cell sarcoma of the abdomen. Calcification within the mass is possibly seen in the extranodal type of the lesion. Further, a follicular dendritic cell sarcoma generally behaves more like a sarcoma than a lymphoma (14). Actually, we detected a small area of direct invasion to the adjacent peritoneum for the case of the colon mass, as was determined from the pathological examination.
In this report, we have described the imaging findings of follicular dendritic cell sarcoma of the abdomen. A follicular dendritic cell sarcoma of the abdomen manifested as a discrete, well-enhancing homogenous mass with internal necrosis and regional lymphadenopathy. Internal calcification may also be seen. Based on these imaging findings, although it is a very unusual tumor, a follicular dendritic cell sarcoma should be considered in the differential diagnosis of a retroperitoneal and colonic mass.
Figures and Tables
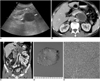 | Fig. 1Follicular dendritic cell sarcoma in 69-year-old man.
A. Abdominal ultrasonography shows 5 cm sized, well-defined anechoic mass (white arrows) that was located in posterior aspect of pancreas (P) with mild mass effect. Mass shows posterior acoustic enhancement that represents necrotic lesion.
B. Axial CT scan shows well-defined necrotic mass with thin peripheral wall in retroperitoneal area (white arrows). Lesion shows mass effect on adjacent pancreas and splenic vein, but there was no evidence of direct invasion.
C. Coronal reconstruction CT image shows several enlarged lymph nodes (black arrows) around cystic mass (white arrows).
D. Gross specimen shows central whitish-yellow color that microscopically demonstrates extensive internal necrosis.
E. Microscopic examination of solid component of mass reveals bundles of ovoid to spindle cells with pinkish cytoplasm (Hematoxylin & Eosin staining, ×100).
|
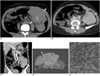 | Fig. 2Follicular dendritic cell sarcoma in 52-year-old woman.
A. Pre-contrast axial CT image shows multiple foci of irregular and dense calcifications in mass (white arrows).
B. Post-contrast axial CT image shows well-defined large mass in mid-descending colon. Mass is composed of intraluminal (white arrow) and extraluminal (black arrow) components. Intraluminal component is less enhanced than extraluminal component.
C. Coronal reconstruction CT image shows several enlarged regional lymph nodes (black arrows) around mass (white arrow).
D. Gross specimen shows intraluminal component with relatively dull yellow color (white arrow) and non-necrotic area of extraluminal component (black arrow) with pink-tan color. Several enlarged pericolonic lymph nodes (asterisks) are noted.
E. Immunohistochemical staining for CD35 demonstrates positive cytoplasmic expression (×400).
|
References
1. Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990. 117:185–211.
2. Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986. 122:562–572.
3. Chan JK, Banks PM, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, et al. A revised European-American classification of lymphoid neoplasms proposed by the International Lymphoma Study Group. A summary version. Am J Clin Pathol. 1995. 103:543–560.
4. Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997. 79:294–313.
5. Kairouz S, Hashash J, Kabbara W, McHayleh W, Tabbara IA. Dendritic cell neoplasms: an overview. Am J Hematol. 2007. 82:924–928.
6. Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol. 2006. 36:249–253.
7. Low SE, Menasce LP, Manson CM. Follicular dendritic cell sarcoma: a rare tumor presenting as an abdominal mass. Int J Surg Pathol. 2007. 15:315–317.
8. Schwarz RE, Chu P, Arber DA. Extranodal follicular dendritic cell tumor of the abdominal wall. J Clin Oncol. 1999. 17:2290–2292.
9. Diaz de Liano A, Garde C, Artieda C, Yarnoz C, Flores L, Ortiz H. Intra-abdominal follicular dendritic cell sarcoma. Clin Transl Oncol. 2006. 8:837–838.
10. Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala AG, Ordonez NG, et al. Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with a review of the literature. Mod Pathol. 2002. 15:50–58.
11. Leipsic JA, McAdams HP, Sporn TA. Follicular dendritic cell sarcoma of the mediastinum. AJR Am J Roentgenol. 2007. 188:W554–W556.
12. Meador TL, McLarney JK. CT features of Castleman disease of the abdomen and pelvis. AJR Am J Roentgenol. 2000. 175:115–118.
13. Chan AC, Chan KW, Chan JK, Au WY, Ho WK, Ng WM. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman's disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology. 2001. 38:510–518.
14. Fonseca R, Tefferi A, Strickler JG. Follicular dendritic cell sarcoma mimicking diffuse large cell lymphoma: a case report. Am J Hematol. 1997. 55:148–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download